Gas Laws and Pure Substances
1/45
Earn XP
Description and Tags
MEE1018: Thermodynamics and Fluid Mechanics - Lecture 2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Temperature
It is a measure of ‘how hot’ or ‘how cold’ something is relative to some reference point.
Temperature difference
The potential to produce heat flow (like voltage). Heat will flow naturally from higher temperature to lower temperature.
Thermal equilibrium
When two objects have the same temperature.
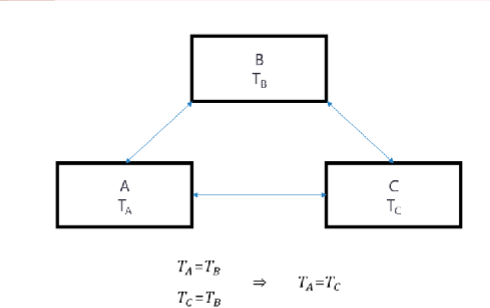
Zeroth Law of Thermodynamics
When two objects are separately in thermodynamic equilibrium with a third object, they are in equilibrium with each other.
Uses of Zeroth Law
Used to calibrate a thermometer by putting the thermometer in thermal equilibrium with a known physical system at several reference points. (e.g. freezing and boiling point of water).
Fahrenheit scale
0F = Freezing point of salt / ice
100F = Body temperature
Celsius scale
0C = Freezing point of water
100C = Boiling point of water
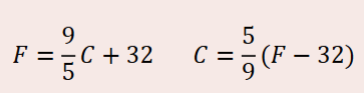
Celsius and Fahrenheit conversion
The gas thermometer
If pressure times volumes is plotted against temperature for any gas is a straight line.
Relationship between temperature and pressure of a gas in a vessel
T = a +bP, where a and b are constants determined experimentally.
Kelvin scale
0 = The lowest theoretical temperature possible. Also called the absolute scale.
Use of Kelvin scale
When doing absolute temperature measurements or taking ratios
Kelvin, Celsius Conversion
T(K) = T(°C) + 273.15
Scale used when measuring temperature differences ΔT
Kelvin or Celsius
Latent heat
The amount of energy absorbed or released during a complete phase change.
Latent heat of fusion
The energy input required to completely melt a solid.
Latent heat of vapourisation
The energy input needed to completely vapourise a fluid.
Gas variables
p: pressure, V: volume, T: temperature, n: moles or m: mass
To investigate the relationship between 2 gas variables
We need to hold the other 2 constants.
Constant p - same number of collisions / unit area (frictionless piston in vertical cylinder).
Constant V - rigid container.
Constant T - thermostat control
Constant n or m - keep container sealed.
Boyle’s Law
The pressure of a gas is inversely proportional to its volume.
p ∝ 1/V pV = C p1V1 = p2V2
Charles’ Law
When the pressure of a gas is constant, the volume will be directly proportional to temperature on the Kelvin scale.
V ∝ T V/T = C V1/T1 = V2/T2
Amonton’s Law / Gay Lussac’s Law
The ratio between the volumes of the reactant gases and the gaseous products can be expressed in simple whole numbers.
p ∝ T p/T = C p1/T1 = p2/T2
Avogadro’s Law
Equal volumes of gases have equal numbers of molecules at equal temperature and pressure.
V ∝ n V/n = C V1/n1 = V2/n2
Ideal gas equation
pV = nR0T, where R0 is the universal gas constant
pV = mRT
Individual gas constant
Unique to each particular gas
Individual gas constant equation
R = R0/M
Other useful forms of the universal gas equation
p = ρRT
pv = RT
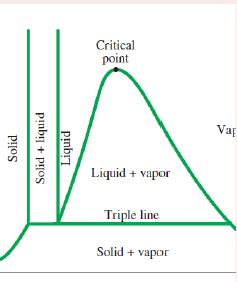
Pure substance
A substance that has a fixed chemical composition throughout.
Pure substance examples
Nitrogen and gaseous air
A pure substance in more than one phase
A pure substance may exist in more than one phase, if the chemical composition is the same in all phases.
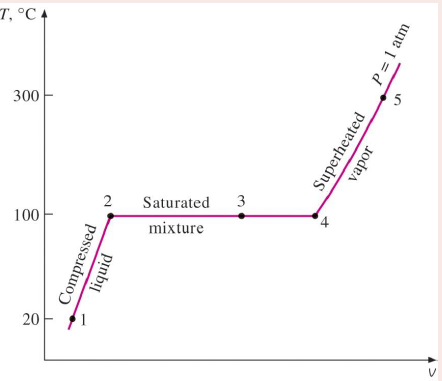
Compressed liquid
A liquid well below boiling point.
Saturated liquid
A liquid that is ready to vapourise.
Saturated liquid-vapour mixture
Part of the saturated liquid is vapourised.
Saturated vapour
The temperature remains constant until the last drop of liquid is vapourised.
Superheated vapour
The temperature of the vapour starts to rise.
T-V graph of a pahse chage processes of pure substances
Water at atmospheric pressure
Saturation temperature, Tsat
The temperature at which a pure substance changes phase at a given pressure.
Saturation pressure, Psat
The pressure at which a pure substance changes phase at a given temperature.
T-V Isobars at different pressures
As pressure increases
Boiling point (saturation temperature) increases
Specific volume of saturated vapour decreases.
The flat regions of the isobars correspond to states for which vapour and liquid coexist, i.e. there is a mixture of gas and liquid.
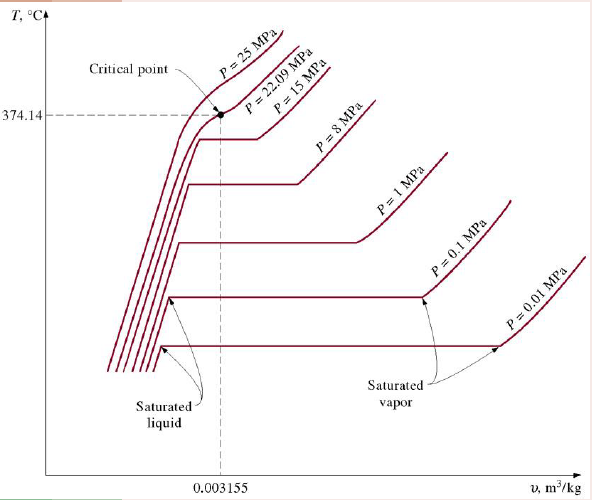
Critical point
The point of inflexion on a t-v graph,
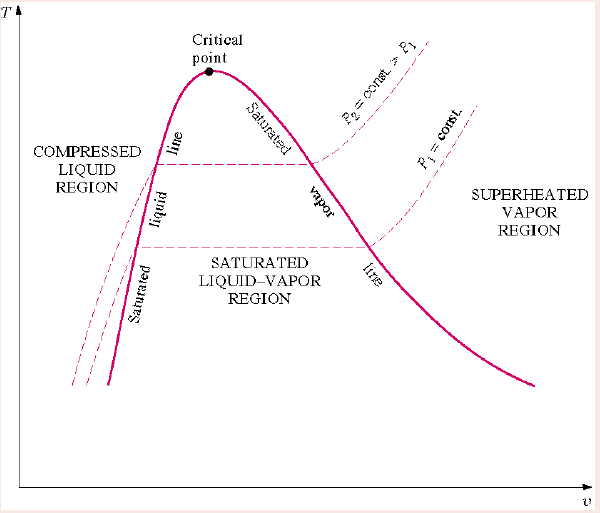
Reading a phase change process diagram
Inside the dome - liquid and vapour present (saturated mixture)
To the left of dome - only liquid present (compressed liquid region)
To the right of dome - only vapour present (superheated vapour / gas region)
Above the critical point - supercritical isobars
Supercritical isobars
Isobars above the critical point.
Supercritical fluid
A material that has properties intermediate between a liquid and a gas. S
Triple point
A point where all three phases coexist (solid, liquid and gas).
Triple point pressure and temperature
A substance exists in three phases in equilibrium (all states at same temperature and pressure, but different specific volumes).
Sublimation
The process in which, below the triple point pressure solids will not melt, i.e. there is no liquid phase, but instead will pass directly from a liquid to a solid.