CHEM 108: Atomic Structure, Ions, Isotopes, Valence Electrons, Bonds, & Periodic Table
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Protons
positive charge, number the same as electrons
- 1 amu
Neutrons
no charge
- 1 amu
Electrons
negatively charged, number the same as protons
- 0.000549 amu
What is amu?
atomic mass unit
Atomic number
how many protons are in an element
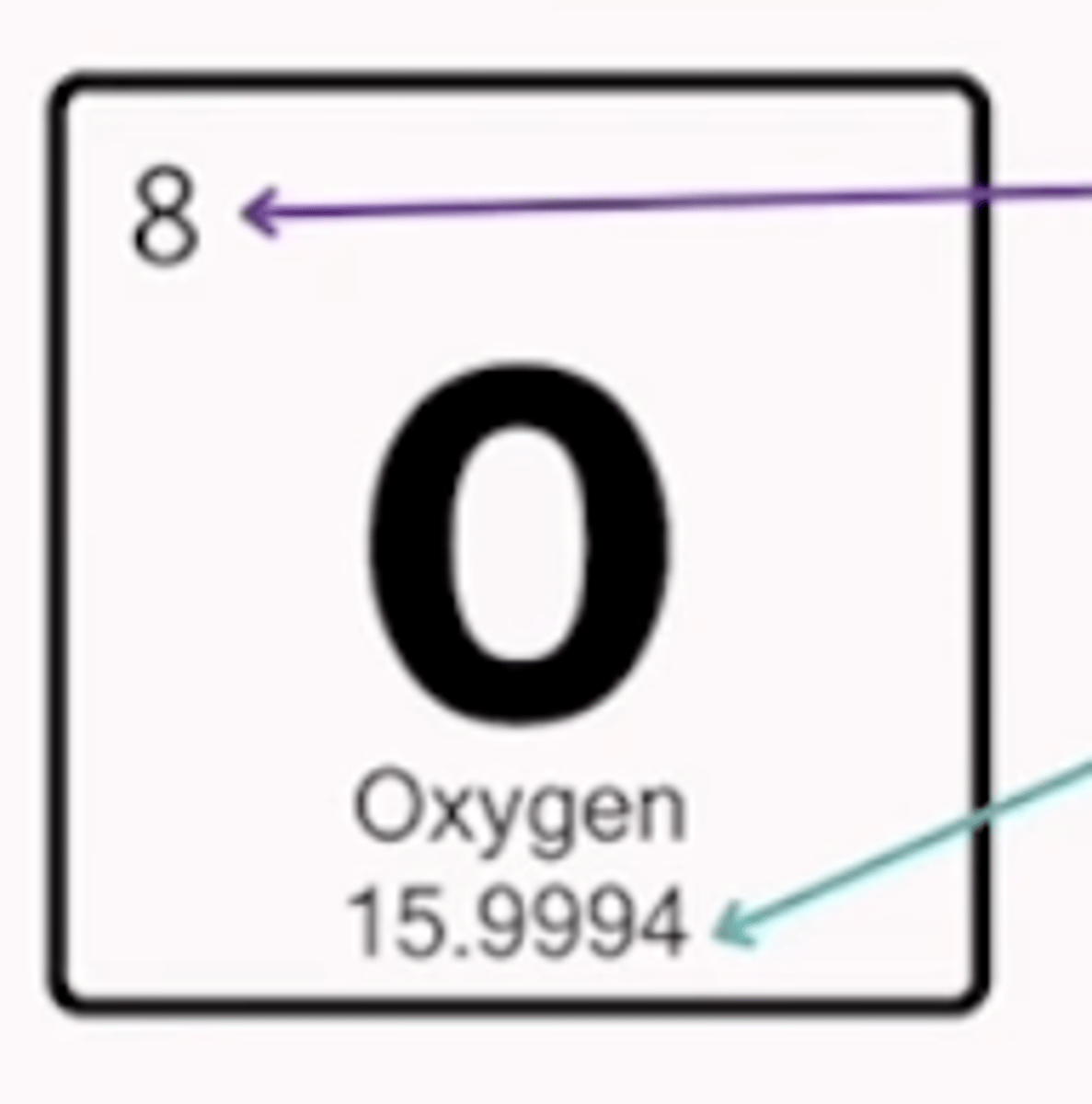
Atomic mass
how many protons and neurons are in an element
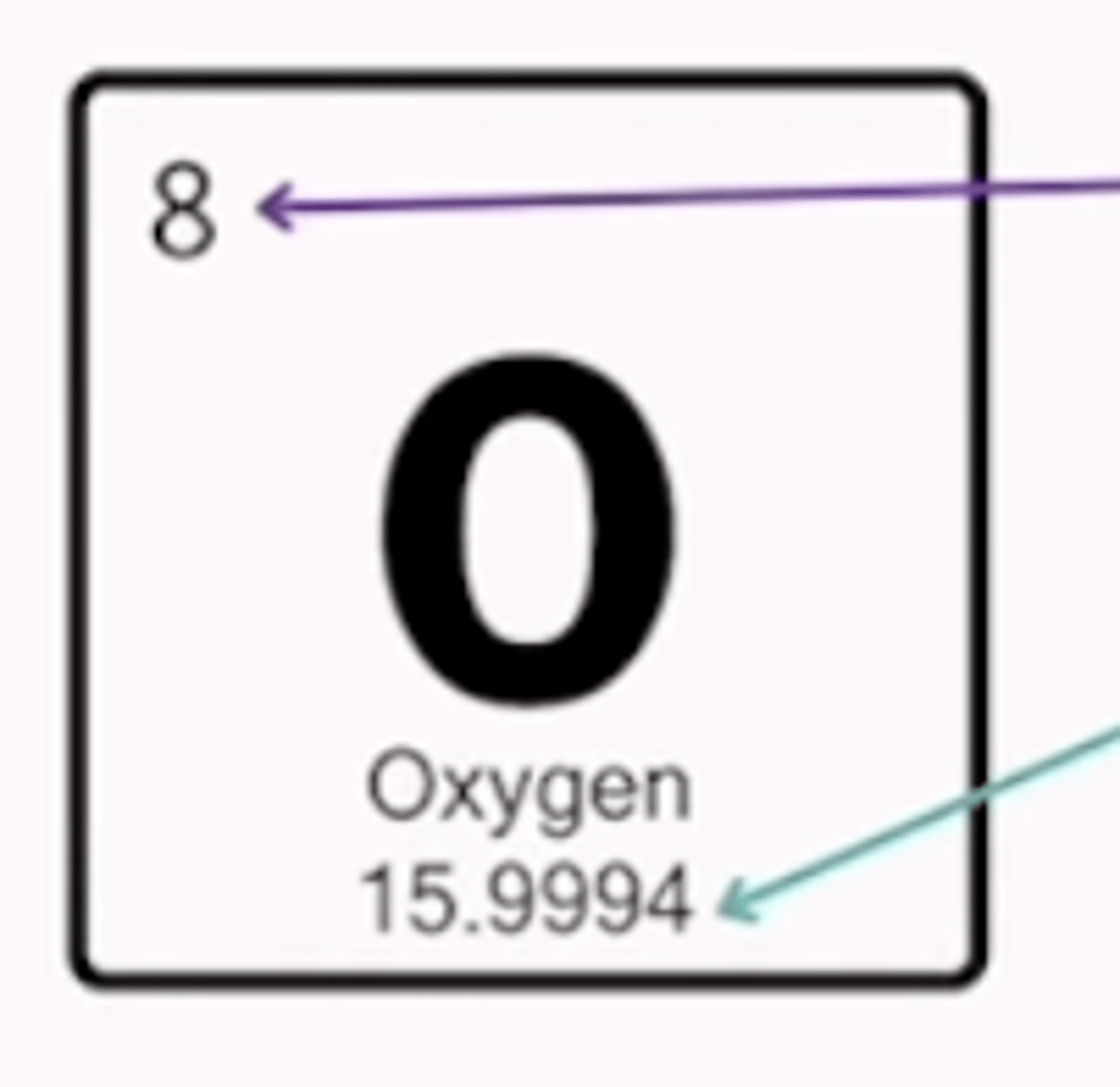
Equation to find neutron number
mass number - atomic number = # of neutrons
Isotopes
same chemical, differ in number of neurons
ion
atom or group of atoms with net electrical charge
How does an atom become an ion?
If an atom gains or loses electrons
cation
positive ion, loses electrons and shows +
anion
negative ion, gains electrons and shows -
Shell
path the electrons revolving around nucleus takes
sub-shell
subdivision of electron shells separated by electron orbital
orbital
3D space in atom where electron in a sub-shell can be found
Octet rule
atoms lose, gain or share electrons to get eight valence electrons
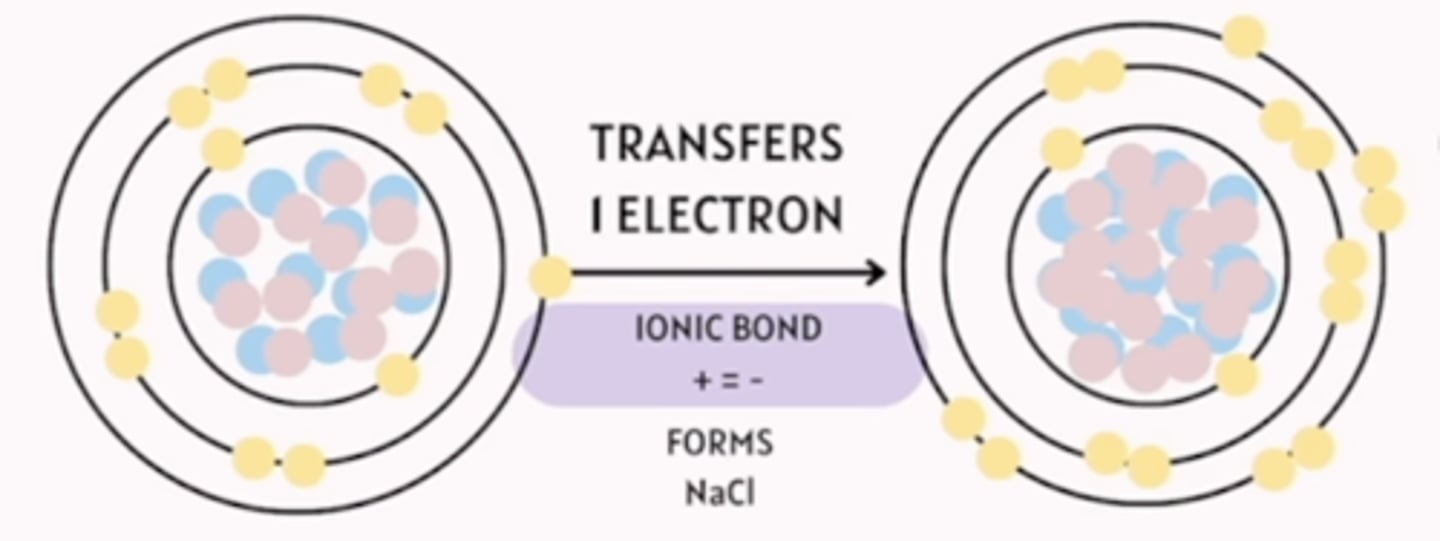
ionic bond
complete transfer of electrons "i give, you take"
covalent bond
sharing of electrons to form electron pairs between atoms "sharing is caring"
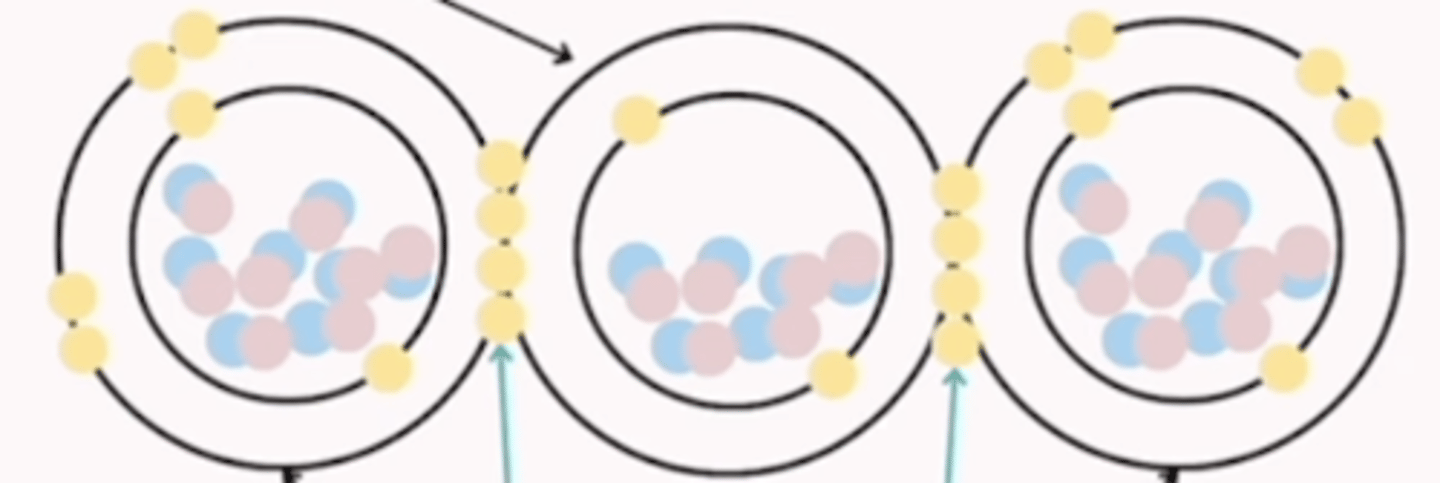
4 major elements that form covalent bonds
carbon, oxygen, hydrogen, nitrogen
What occurs during the formation of an ionic bond?
electrons are transferred from one atom to another
Describe a covalent bond
atoms share a pair of electrons
The octet rule primarily influences and atom's...
electron configuration
What do all elements in the same period on the periodic table all have in common
The share the same number of electron shells
What do all elements in the same row on the periodic table have in common?
The have similar chemical properties