IR Spectroscopy Images
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Aromatic
- C-H Stretch 3100-3000
- C=C Stretch 1620-1440
- C-H Bend 900-680

Ether
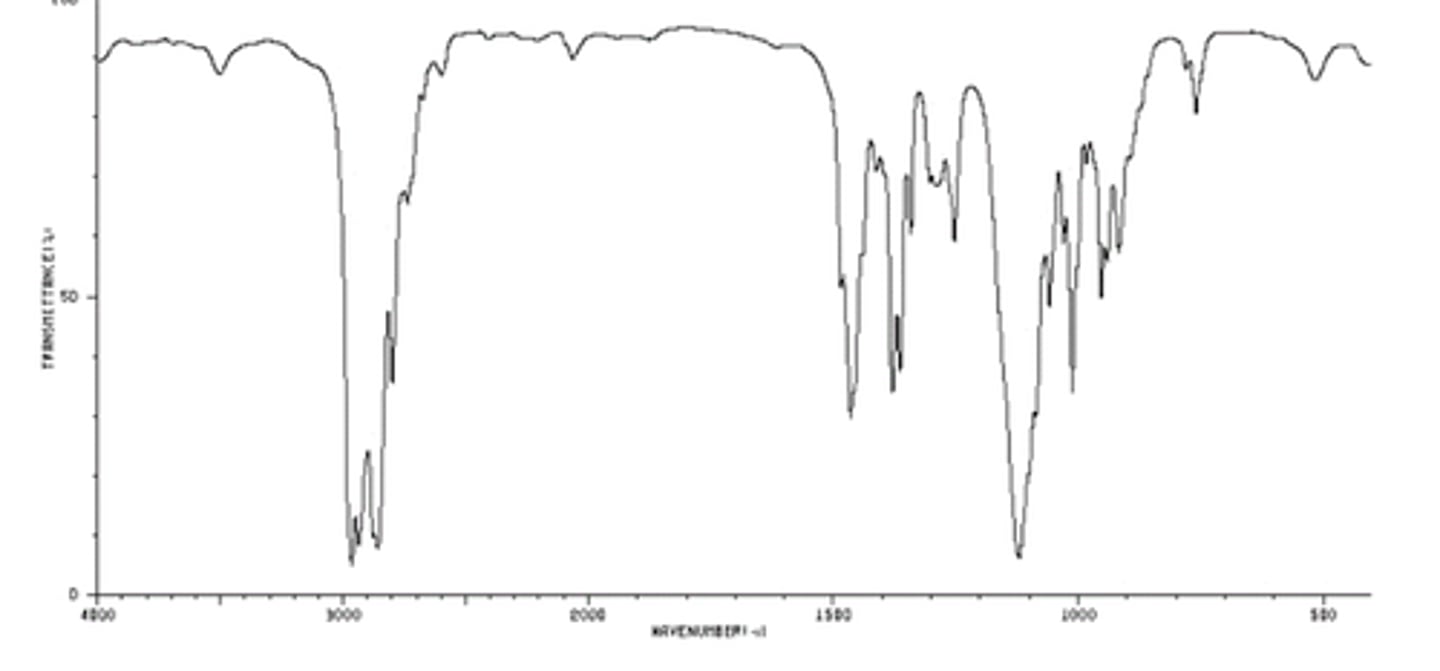
Ester
- Strong C=O stretch (1765-1735 (sat.), 1730-1715 (conj.))
- strong C-O stretch
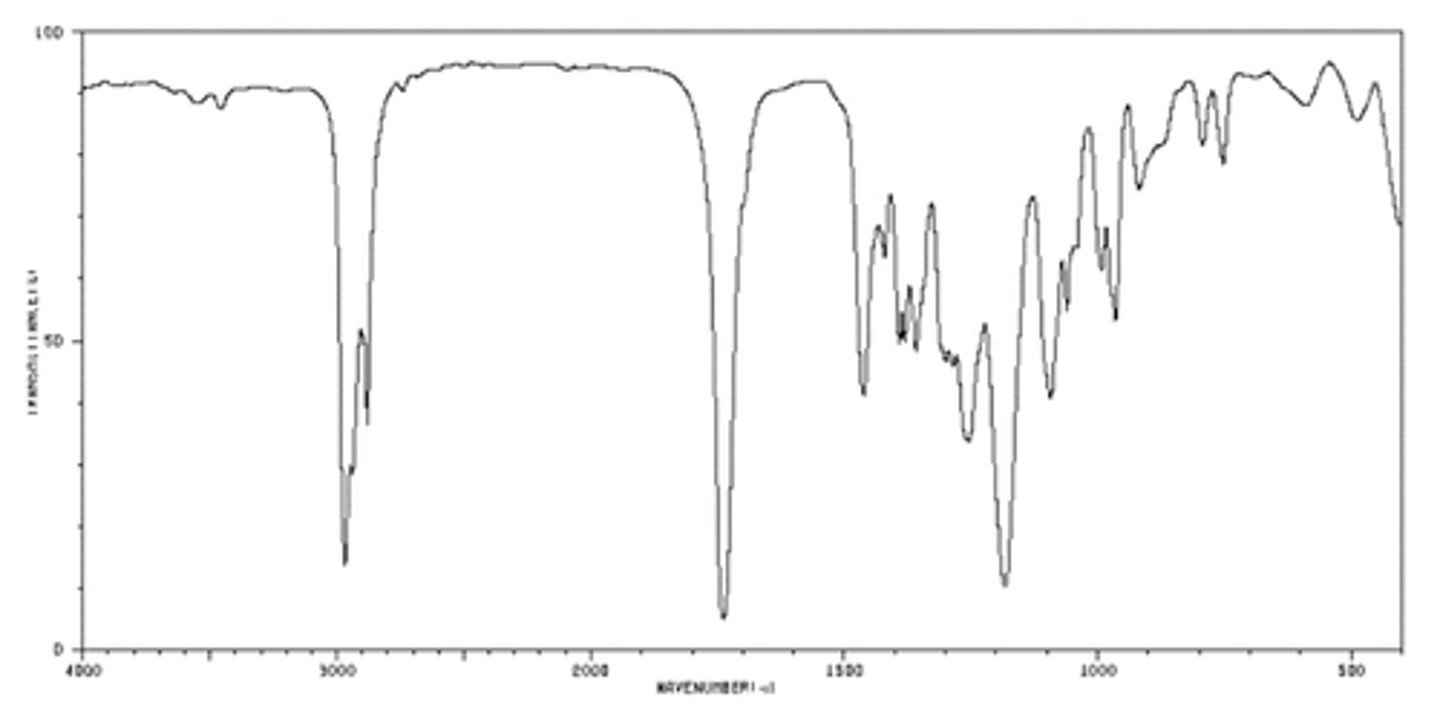
Primary amine
N-H stretch around 3300-3500
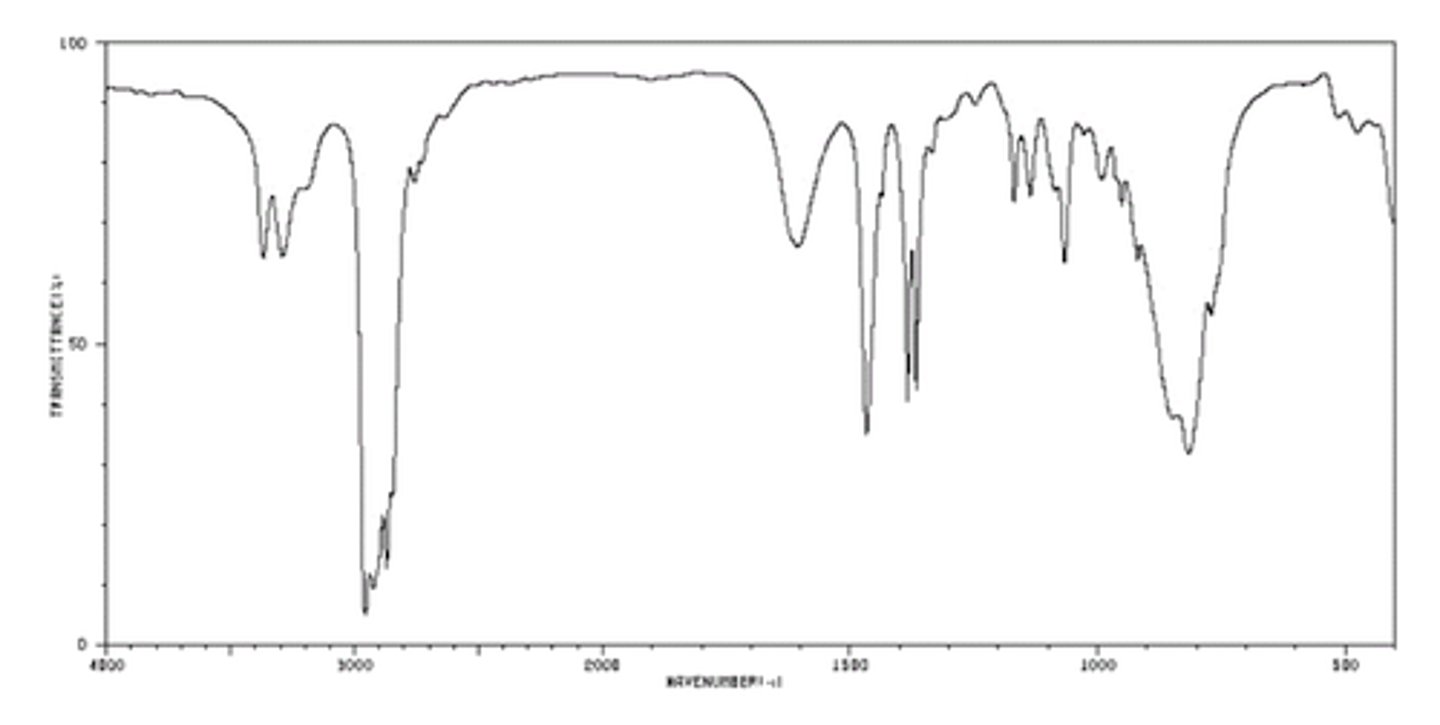
Ketone
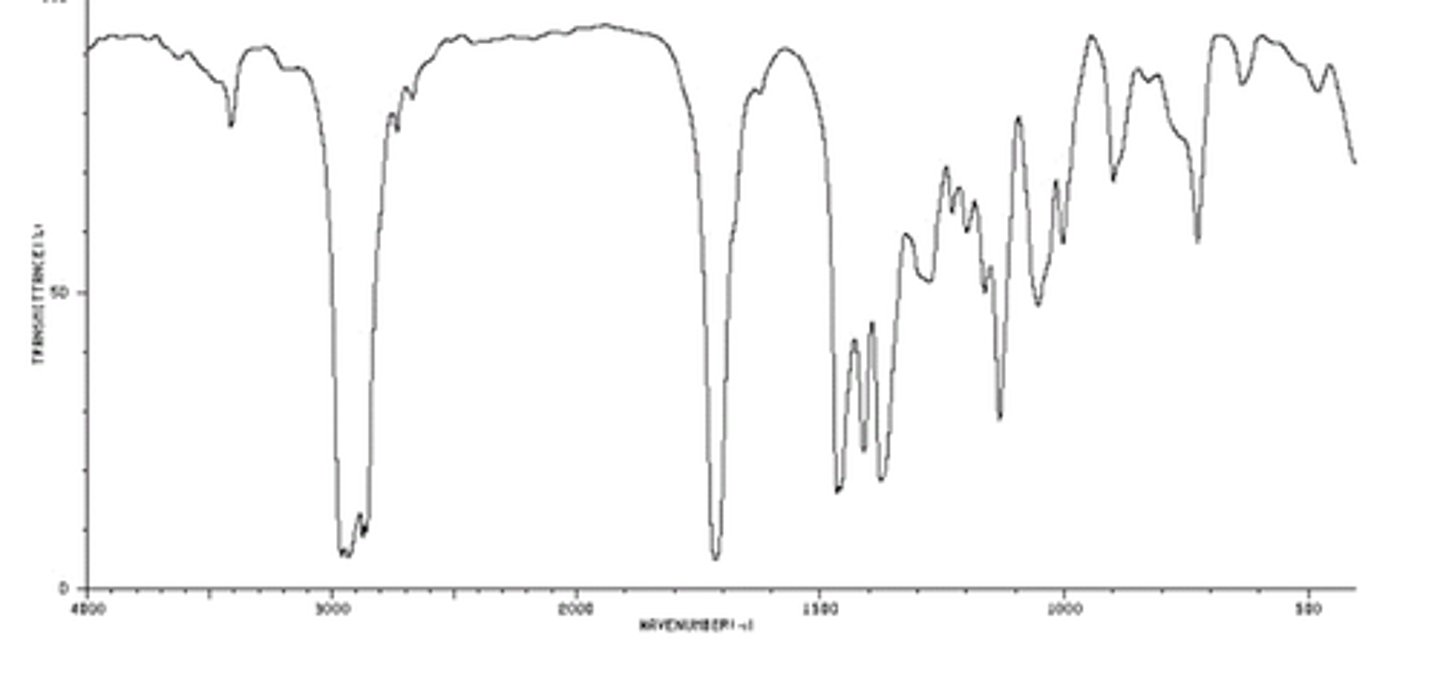
Aldehyde
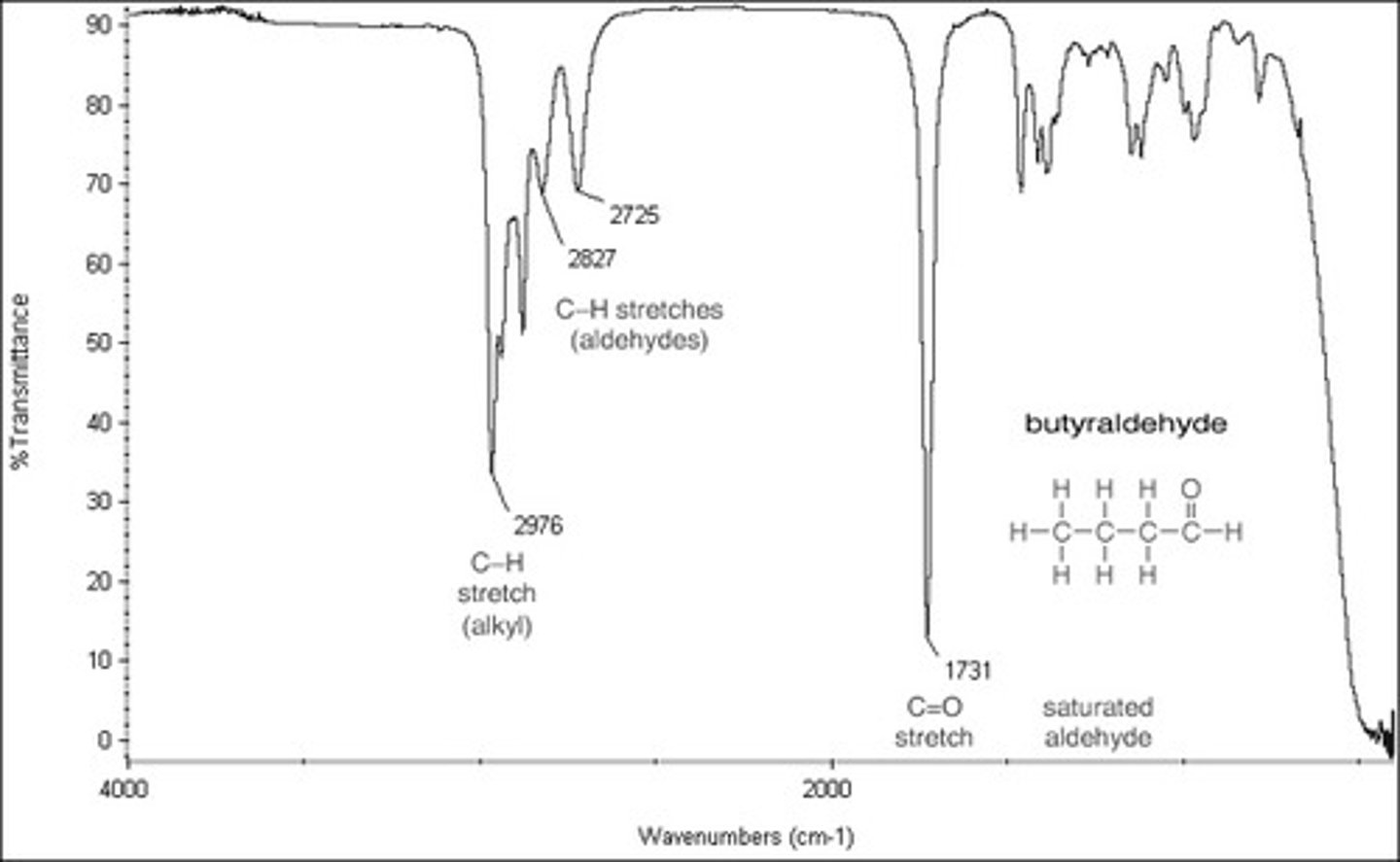
Alcohol
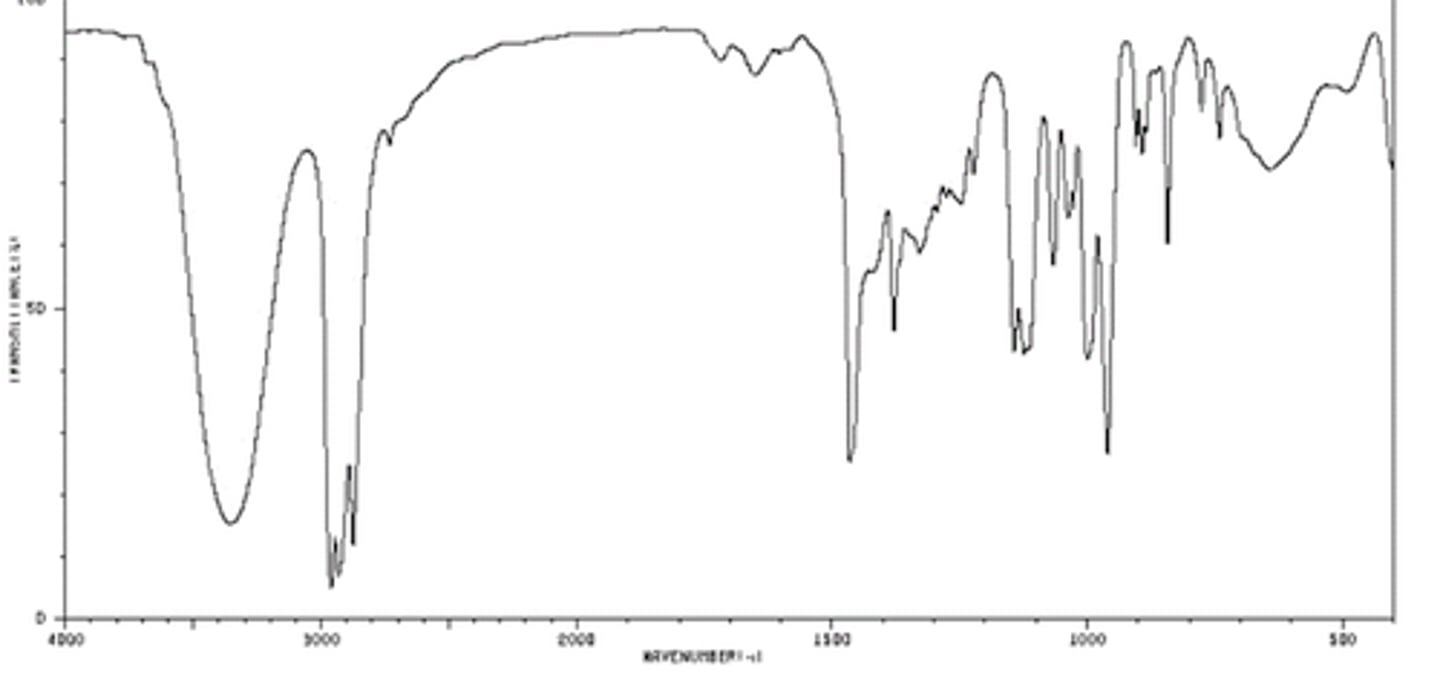
Carboxylic Acid
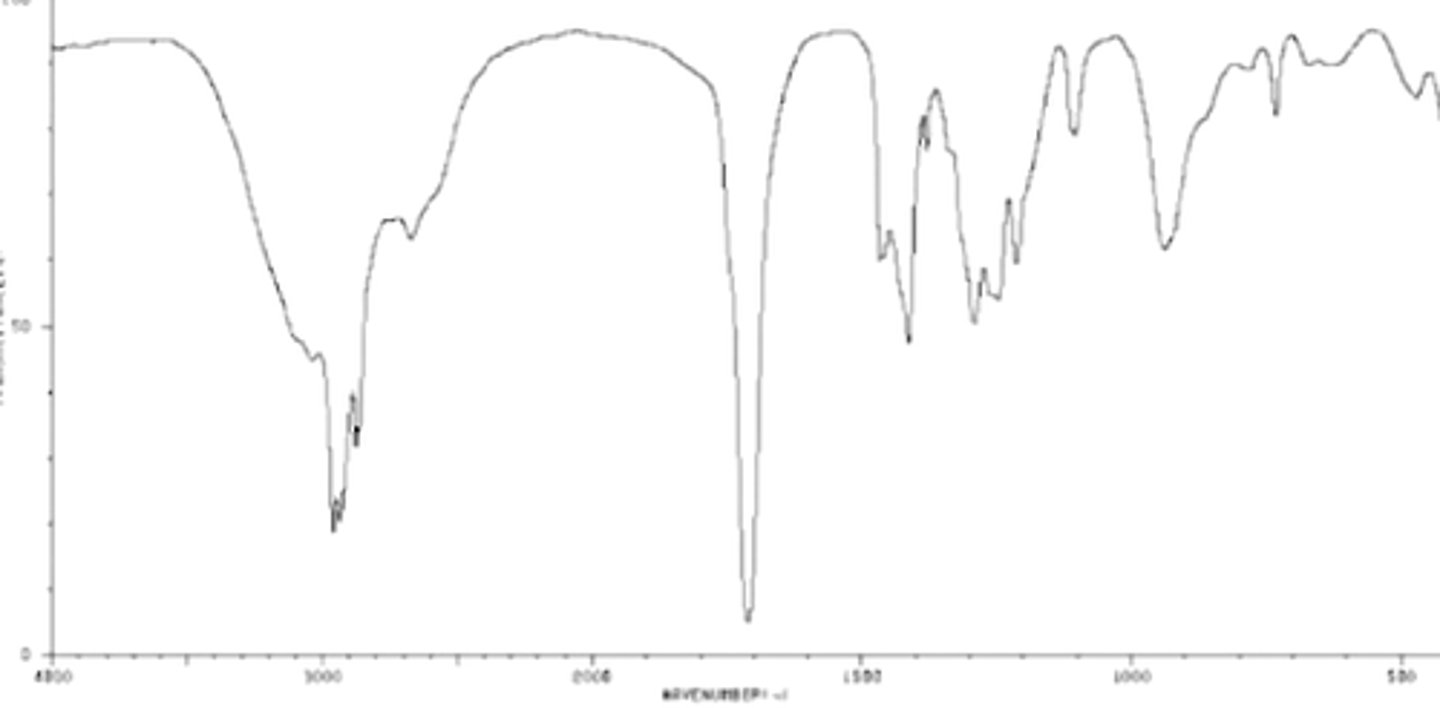
Amide
The two double peaks around 3200 and 3400 are two single bond H on a N,
Double peak between 1600-1700 almost represent a carbonyl but are too far right
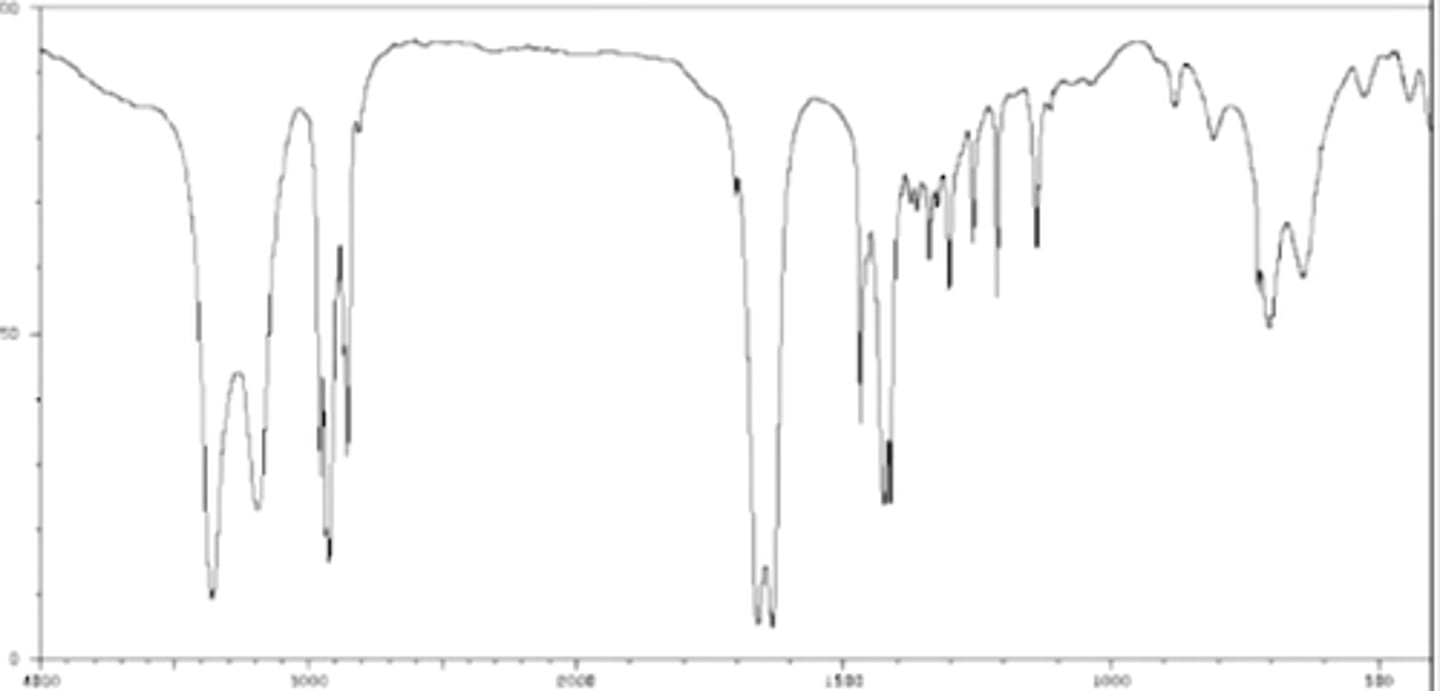
Alkyne
Sharp but medium peak at ~2100
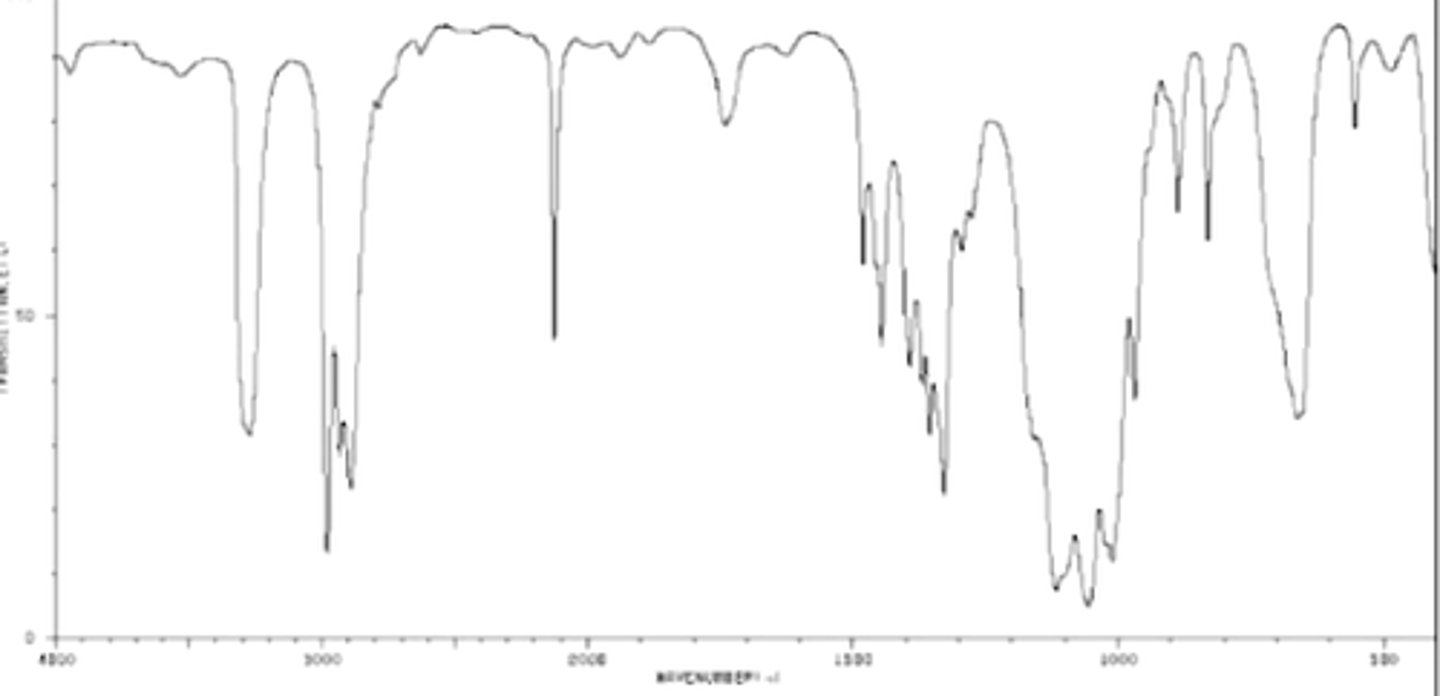
What Functional Group has a formula of C5H10O2, an intense stretch at 1715, and a very broad stretch centered at ~3100 cm-1?
AI Overview
The functional group is a
carboxylic acid.
Here's why:
Molecular Formula: C5H10O2 suggests a compound with two oxygen atoms and likely contains a functional group involving both carbon and oxygen.
IR Spectrum:
Intense stretch at 1715 cm⁻¹: This is characteristic of a carbonyl (C=O) group. This stretch is typically strong and sharp due to the high dipole moment of the C=O bond. The range for carbonyl stretches in carboxylic acids is generally 1700-1725 cm⁻¹.
Very broad stretch centered at ~3100 cm⁻¹: This indicates an O-H stretch, and its broadness suggests hydrogen bonding. This is a hallmark of carboxylic acids. Carboxylic acids tend to form hydrogen-bonded dimers, leading to a very broad O-H stretch. The characteristic O-H stretch for a carboxylic acid is found in the range of 2500-3300 cm⁻¹.
The presence of both a carbonyl group and a broad O-H stretc
What Functional Group has a formula of C5H9N and stretches at just below 3000 cm-1 and a stretch at 2250 cm-1?
Step 1 . Analyze the C-H stretch
The stretch just below
3000cm-13000 c m to the negative 1 power
3000𝑐𝑚−1
indicates the presence of
sp3s p cubed
𝑠𝑝3
hybridized C-H bonds.
Step 2 . Analyze the stretch at
2250cm-12250 c m to the negative 1 power
2250𝑐𝑚−1
The stretch at
2250cm-12250 c m to the negative 1 power
2250𝑐𝑚−1
indicates the presence of a nitrile group (
−C≡Nnegative cap C triple bar cap N
−𝐶≡𝑁
).
Step 3 . Combine the information
The molecular formula
C5H9Ncap C sub 5 cap H sub 9 cap N
𝐶5𝐻9𝑁
and the presence of a nitrile group suggest that the functional group is a nitrile with a four-carbon chain.
Solution
The functional group is a nitrile.
What Functional Group has a formula of C6H10O with stretches at just below 3000 cm-1 and a strong broad stretch centered at 3350 cm-1.
Step 1 . Analyze the IR stretches
The stretch just below 3000 cm$^{-1}$ indicates C-H bonds.
The strong, broad stretch at 3350 cm$^{-1}$ indicates an O-H bond.
Step 2 . Determine the functional group
The presence of both C-H and O-H stretches suggests an alcohol.
Given the molecular formula C$_{6}
Hcap H
𝐻
_{10}$O, the functional group is a cyclic alcohol.
Solution
The functional group is a cyclic alcohol.
What Functional Group has a formula of C4H8O2 and an intense stretch at 1737 cm-1.
The functional group that has the formula C4H8O2 and an intense infrared (IR) stretch at 1737 cm-1 is most likely an ester.
Here's why:
The molecular formula C4H8O2 suggests functional groups like carboxylic acids or esters, as they commonly contain two oxygen atoms and have one degree of unsaturation (likely a C=O bond).
The intense stretch at 1737 cm-1 is characteristic of a carbonyl (C=O) group.
Specifically, the C=O stretch for saturated aliphatic esters appears in the wavenumber region of 1750 to 1735 cm-1. The given value of 1737 cm-1 falls directly within this range.
While carboxylic acids also contain a C=O stretch, their typical range for the dimer form is around 1710 cm-1, although they can be higher, particularly for the monomeric form. However, the value of 1737 cm-1 is highly characteristic of an ester.
What Functional Group has a formula of C5H10O2, an intense stretch at 1715, and a very broad stretch centered at ~3100 cm-1?
carboxylic acid
What Functional Group has a formula of C7H12O and an intense stretch at 1724 cm-1 and stretches of moderate intensity at 2746 and 2733 cm-1
What Functional Group has a formula of C7H12O and an intense stretch at 1724 cm-1 and stretches of moderate intensity at 2746 and 2733 cm-1