Environmental Engineering Exam 1
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
What is environmental science?
Environmental science is the interdisciplinary study of the interactions between the physical, chemical, and biological components of the environment. It involves understanding human impact on ecosystems and developing strategies for sustainable management.
What is environmental engineering?
Engineering for the purpose of environmental protection, public health, and sustainability.
Applies math and science to utilize the properties of matter and sources of energy in the solution of problems relating to sanitation and ecological preservation.
Silent Spring
A groundbreaking book by Rachel Carson published in 1962 that raised public awareness about the dangers of pesticide use and its impact on the environment.
PFAs : Poly-/perfluoroalkyl substances
are synthetic chemicals used in various industrial and consumer products, known for their persistence in the environment and potential health risks.
PFA solution
Municipal scale: adsorption, foam fractionalization, and nanofiltration
Destructive methods: UV-based oxidation and reduction, electrochemical and photocatalytic, plasma reactors, and adsorption followed by destructive regeneration.
Environmental Matrices
are the various components of the environment, such as air, water, soil, and organisms, that can be affected by pollutants and other environmental factors.
They serve as mediums for pollutant transport and interaction.
Water matrices play a central role in environmental science because:
Historical use of water bodies as disposal and dilution tools
Dangers posed by contaminated water
Water use in industrial processes, household, and transportation
Rapid transport of contaminants in waterways and hydraulic cycling.
Unusual properties of water
Density (expands when frozen; most dense at 4* C), Melting/boiling points (unusually high boiling and freezing point relative to its molecular weight), Specific heat (very high 4184 J/kg·K), heat of vaporization (very high) Solvent properties (excellent solvent for many substances), Greenhouse effect (due to vibrational transitions, it absorbs infrared radiation)
The Hydrologic Cycle
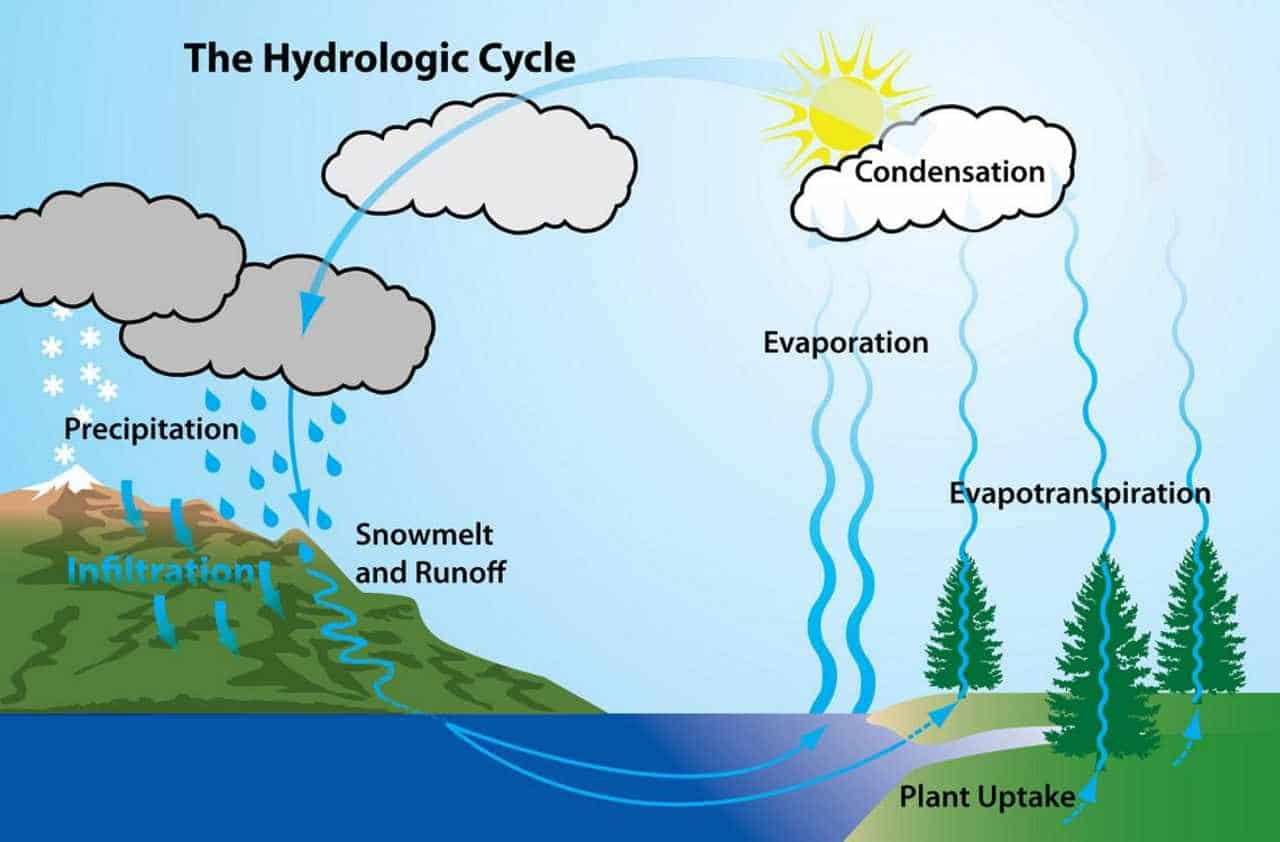
Water stores on earth
-World Oceans: 96.5%
-Saline groundwater : 1%
-Fresh groundwater : 0.76%
-Antarctic glaciers : 1.56%
-Greenland glaciers : 0.17%
water stores on earth
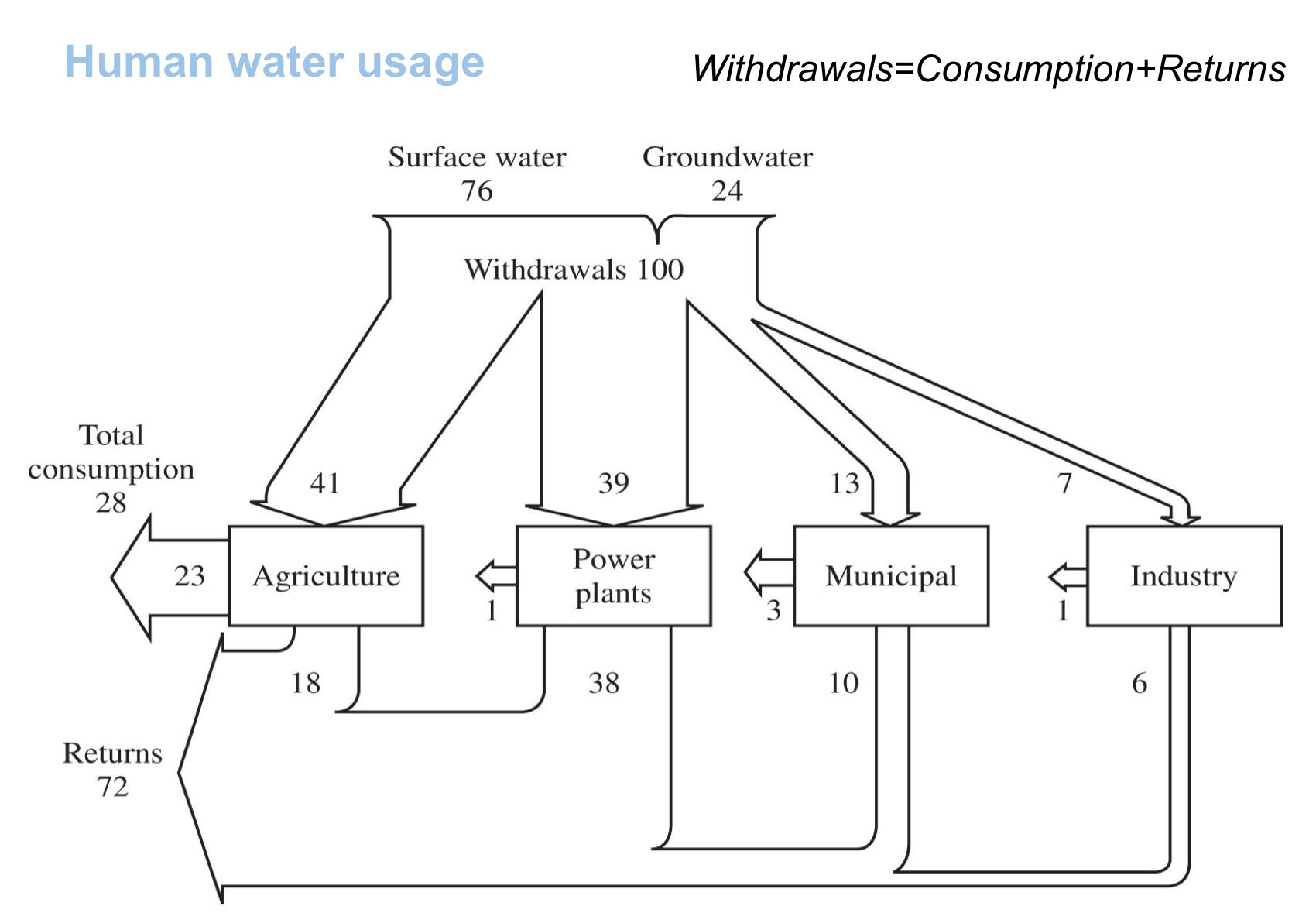
Human water usage
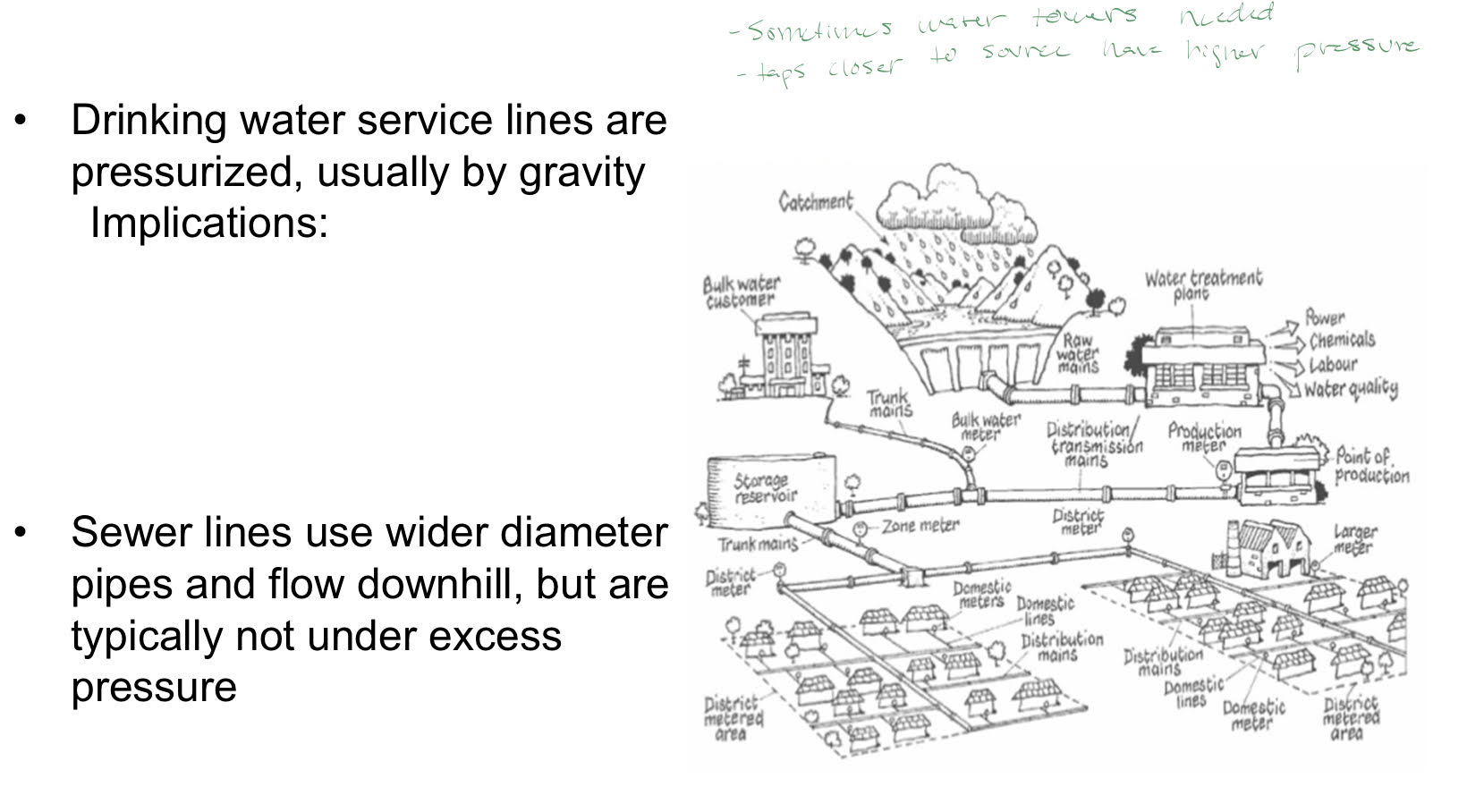
Water and Wastewater systems
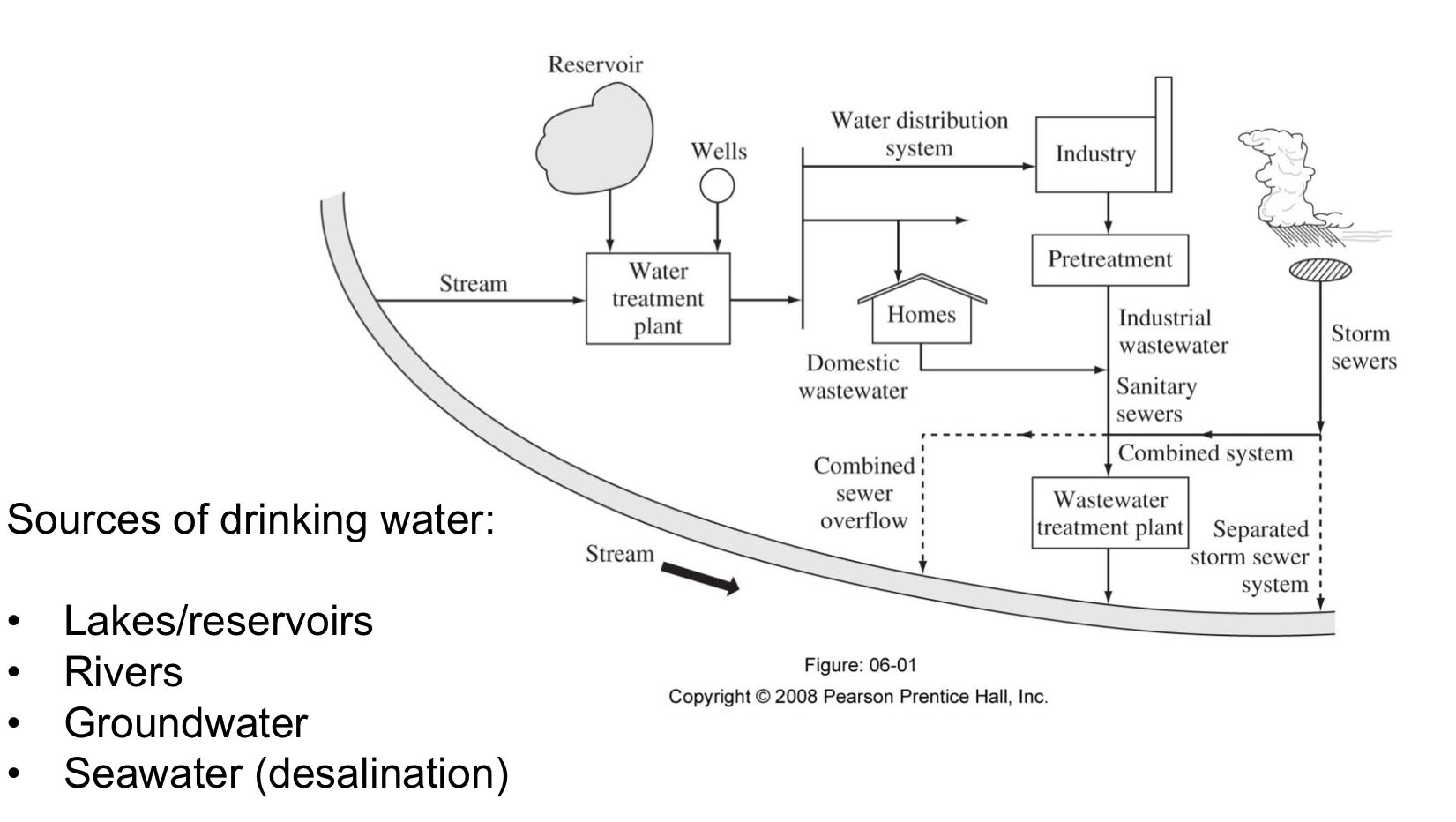
Distribution and collection
Water pollutants
Pathogens, oxygen-demanding wastes, nutrients, salts, heat, heavy metals, pesticides, volatile organic chemicals, and emerging contaminants.
Dangers of DHMO ( Dihydrogen Monoxide )
death due to inhalation, prolonged exposure leads to severe tissue damage, over-ingestion side effects, acid rain component, severe burns, metal corrosion, etc.
The Clean Water Act (1972)
Goal: to make water “fishable and swimmable”, made it unlawful to discharge any pollutant from a point source into navigable waters unless permit obtained, regulates what can be discharged into surface waters
Point Source Pollution
discharged from a discrete point, such as a pipe or smokestack. Easier to regulate, treat, and control.
Nonpoint Source Pollution
contamination as a result of contaminated rain, runoff, or snowmelt. Not subject to permitting under the CWA
Solid Water Contaminants
The presence of suspended solids is an important indicator of chemical and microbial contaminations, Mineral and organic solids can be problematic themselves, relating to: aesthetics of drinking water, Siltation (aesthetics of surface water, ecological harm, and lake fill-in), interference with water treatment process
Turbidity
a measure of light scattering by particles in the water (NTU)
Total suspended solids (TSS)
sample is filtered, recovered solids are dried and weighed (mg/L)
Waterborne Pathogens
most imp. obj. in drinking water treatment is the removal or inactivation of pathogenic microorganisms, many classifications of microbes can persist in water and cause disease.
Vibrio Cholerae
Cholera: restricted largely due to developing world, rod-shaped, Contaminated water, shellfish, small intestine- causes fluid loss up to 20 L a day
Legionella pneumophila
A form of respiratory pneumonia, prefers warm water, spreads through inhalation of aerosols, an opportunistic pathogen: normally doesn’t cause disease, can effect people with compromised immune systems
Giardia
Flagellated protozoan, in active trophozoites or infective cysts, infects intestines causing severe diarrhea and cramps, removed through filtration
Cryptosporidium parvum
forms highly resistant oocysts, major consideration for drinking water treatment, shows greatest chlorine tolerance, removed by filtration or UV
Waterborne viral pathogens
examples: Norovirus, Adenovirus, Hep. A
traits: chlorine disinfection varies, resistant to UV, filtration by + Cl2 is effective
Schistosoma
animal pathogen, nematode - “blood fluke”, involves snail and mammalian hosts, infection in humas causes Schistosomiasis
Biofouling
example: bacteria clogging a reverse osmosis membrane element
Atom
indivisible unit containing at least one proton, and usually neutrons and electrons
Element
a type of atom with a unique # of protons
Isotopes
variations among a single element having different numbers of neutrons
Ions
atoms having fewer or more electrons than protons, and thus a positive charge (cation) or negative charge (anion)
Molecule
two or more atoms bonded together with either a covalent or ionic bond
Chlorine
The element chlorine (Cl) is a type of atom having 17 protons. Commonly has 18 electrons in nature, existing as the chlorine anion (Cl-). Can be oxidized (loses an electron) to form elemental chlorine radicals (Cl.), which quickly combine to form a covalent molecule, dichlorine (“chlorine gas”), Cl2. Can also exist in a cationic state (e.g. Cl 5+)
Ionic Bond
atoms bound by electrostatic attraction between ions of opposite charge
Covalent Bond
when two or more atoms share electrons
Metallic Bond
bonding characterized by a delocalized electron cloud
Hydrogen Bond
an electrostatic attraction between partially charged hydrogen atoms and an electronegative atom
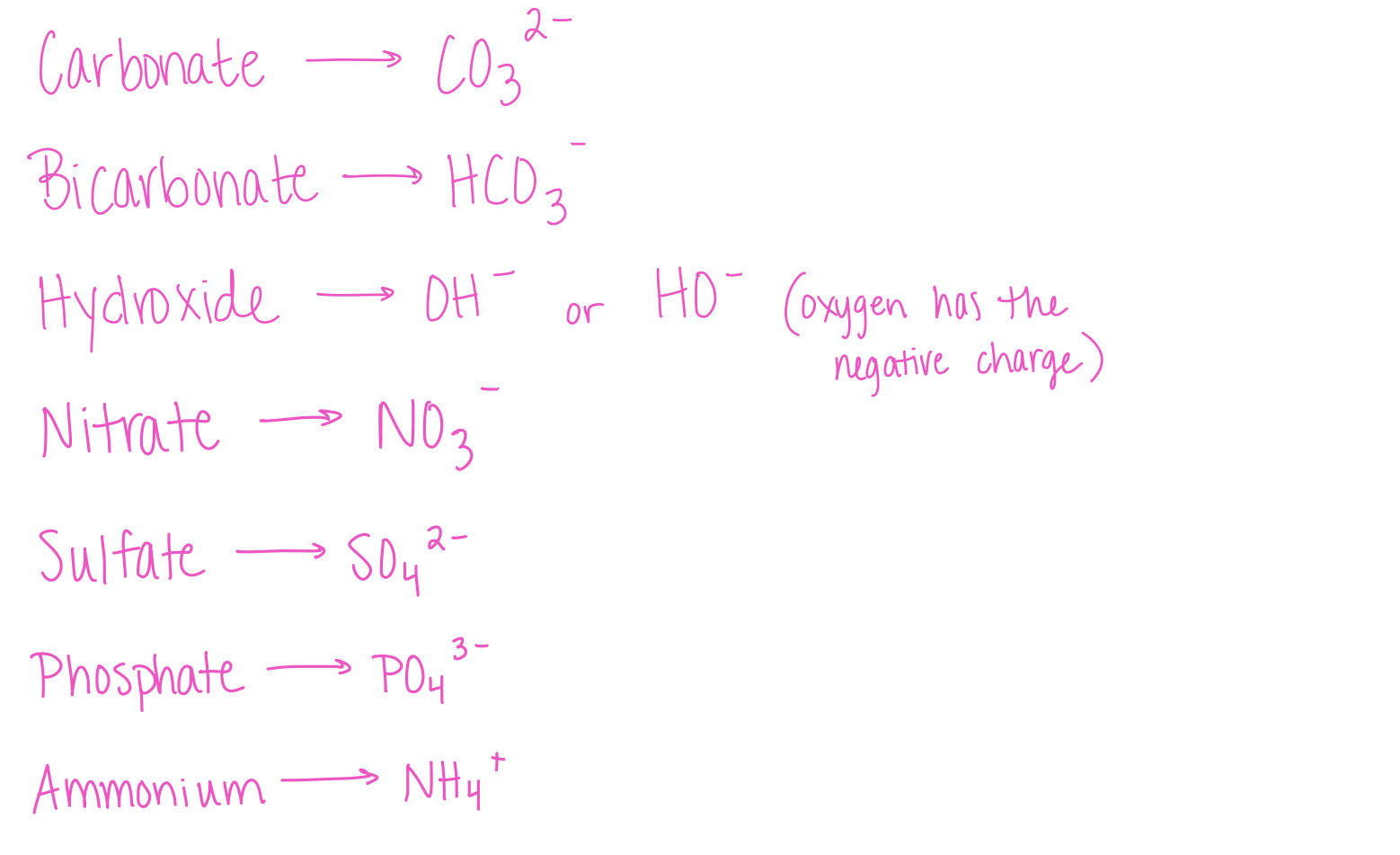
Polyatomic Ions
ions consisting of two or more covalently bound atoms. typically remain as a single unit when dissolved in water. Example: carbonate Ion (CO3 2-)
Important Polyatomic atoms in the environment
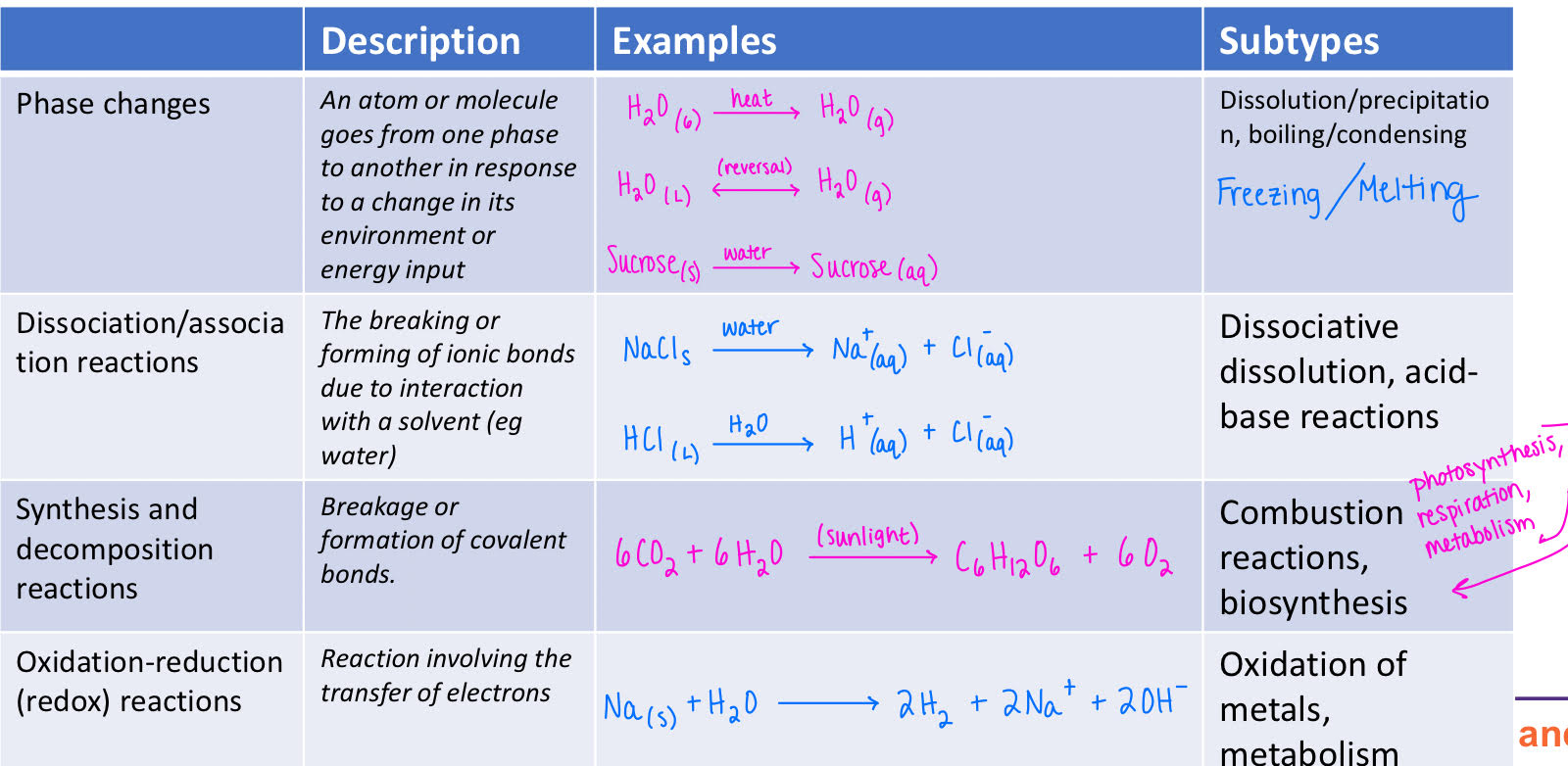
Important Chemical Transformations
Solvent vs Solute vs Solution
vent: a liquid in which substances can dissolve
ute: a compound that dissolves into the solvent
ution: a mixture of a solvent and one or more dissolved solutes
Ionic solutions
always equal amounts positive and negative charges, formed by dissolution of salts, acids, or bases. results in dissociation: the breakage of ionic bonds and formulation of separate dissolved cations and anions stabilized by interaction with polar water molecules.
Molecular Solutions
contain dissolved neutral molecules that disperse from one another but remain intact as a molecule
Dissociation of water
The process by which water molecules break apart into hydrogen ions (H+) and hydroxide ions (OH-) in a reversible manner, there is no pure H2O
The pH scale and H+
the concentration of H+ in water is described using pH, pH = -log[H+] , the pH of pure water is 7 ( [H+] = 10-7 , low pH means acidic (high H+)
pH values
pH < 7 : “acidic water”, Acid: a compound that donated H+ to the solution (dec. pH), strong acid: sulfuric, hydrochloric
pH > 7 : “basic or alkaline water”, Base: a compound that accepts protons or donates hydroxide [H-] (increases pH) strong base: sodium hydroxide
pH : Who cares?
affects: solubility of salts and metals, reaction chemistry, surface charge of solid particles, the conformation of enzymes and integrity of tissues and biomolecules
Oxygen-demanding wastes
When air and water come in contact, molecular oxygen gas, O2(g), will dissolve into the aqueous phase O2(aq)
-healthy surface waters need dissolved oxygen
-leaching of metals; reduced metals tend to be more soluble
Eutrophication
“well fed”, enriched in nutrients, results in algal blooms
-algae essentially covert CO2 into organic carbon via photosynthesis, thus creating potentially oxygen demanding compounds
-bacteria feed on algal byproducts and dead algae, thus consuming O2
Limiting Nutrient
nutrient which limits further growth of plant and microbial populations in an ecosystem, and addition of which may cause eutrophication
-Typically N for seawater
-Typically P for freshwater
Salts and Salinity
-Quantified as Total Dissolved Salts, TDS (mg/L)
-Naturally occurring in inorganic cations; Na+, Ca2+, Mg2+, Sr2+,Fe2+&3+, K+
-naturally occurring in inorganic anions; Cl-, HCO3-, CO32-, SO42-, BO33-
-Seawater 30,000-34,000 mg/L
-Drinking water standard < 500 mg/L
Commons TDS’s
Caspian sea = 12,000 mg/L
Great Salt Lake = 230,000 mg/L
Dead sea = 330,000 mg/L
-Fresh water < 1500 mg/L
-Brackish water 1500 < TDS < 5000 mg/L
-Saline water > 5000 mg/L
“Heavy” Metals
-Most metals can be toxic
Toxicity can depend on the oxidation state(charge)
-Some metals are essential nutrients; Fe and Cr
need low concentration for life, but high conc. toxic for kidneys
-Nephrotoxins : Cd, Pb, Hg, and U
-Metals are non-degradable in the environment
Lead (Pb)
-Sources of water contamination
Legacy lead-soldered plumbing and lead service lines
Coal ash
Runoff from shooting ranges
Atmospheric deposition from polluted air
-Health risks
lead poisoning
Exposure of children to even low levels can result in developmental problems later in life
-EPA action level for drinking water: 15 ppb
Mercury (Hg)
-Sources of water contamination
atmospheric deposition from polluted air
Coal ash
-Health Risk
Typically doesn’t occur in water at hazardous conc. however it bioaccumulates: accumulates in biological tissues and increases in concentration up the food chain
Consuming too much fish can potentially result in mercury poisoning
Arsenic (As)
-Sources of water contamination
Naturally occurring in some groundwater
Coal ash
-Health Risk
Chronic exposure associated with skin, bladder, and lung cancer
EPA MCL for drinking water is 10 ppb
Organic Compound/Molecule
a molecule containing one or mor carbon atoms covalently bonded to other atoms ( most commonly hydrogen and oxygen )
Organic Compound Depictions
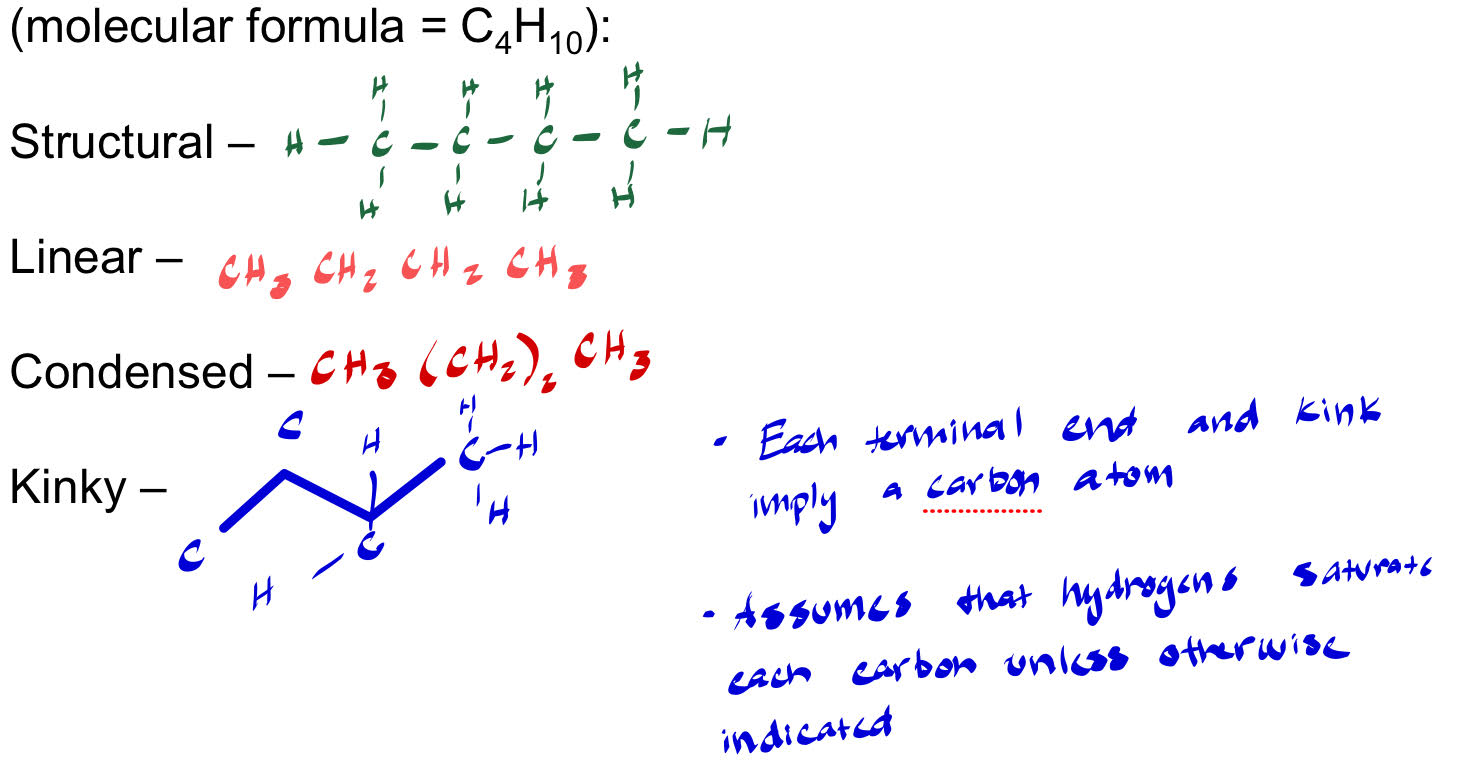
Organic Compounds
Methane (CH4)
Ethane (C2H6)
Propane (C3H8)
Butane (C4H10)
Pentane (C5H12)
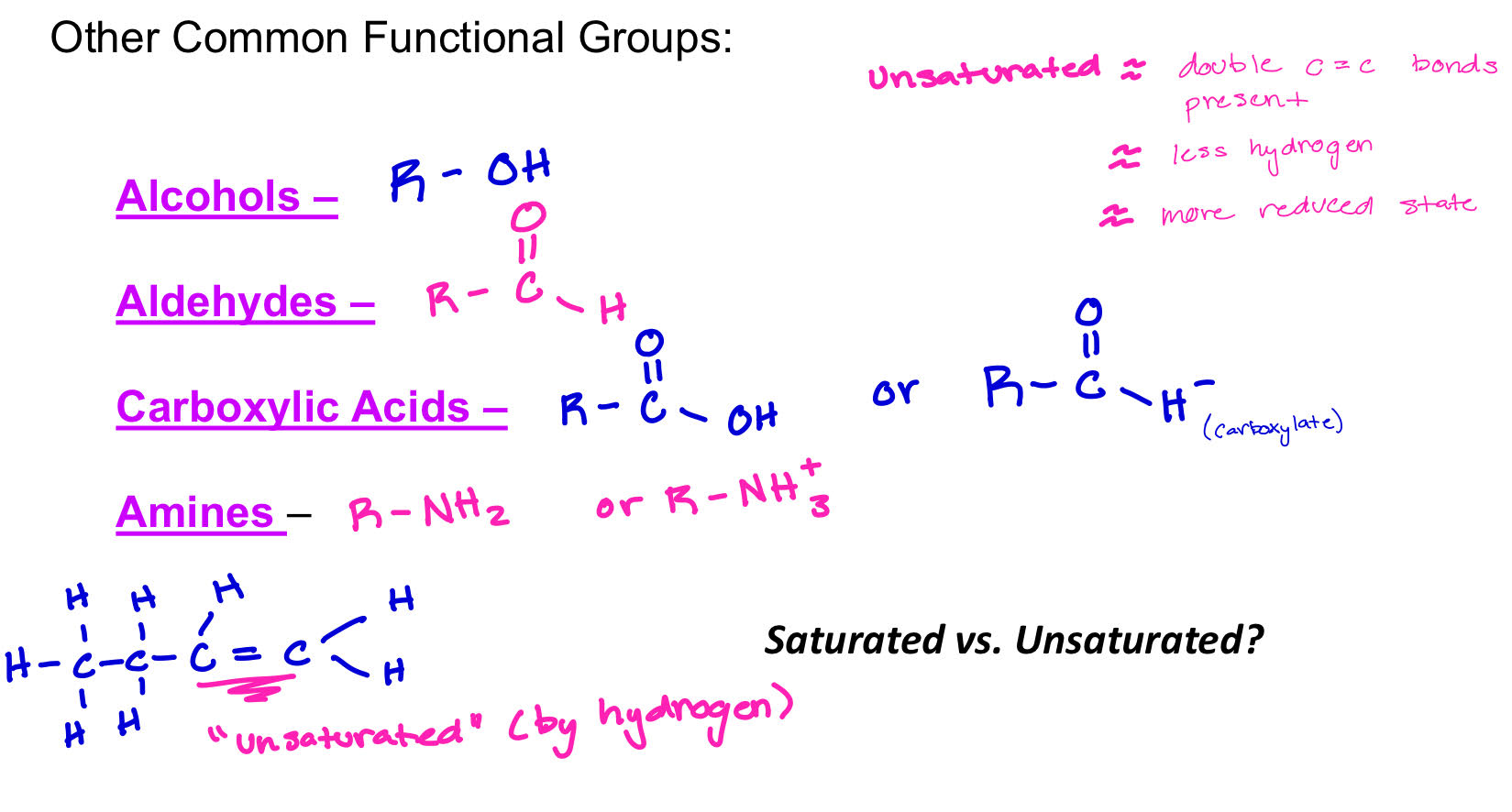
Common Functional Groups
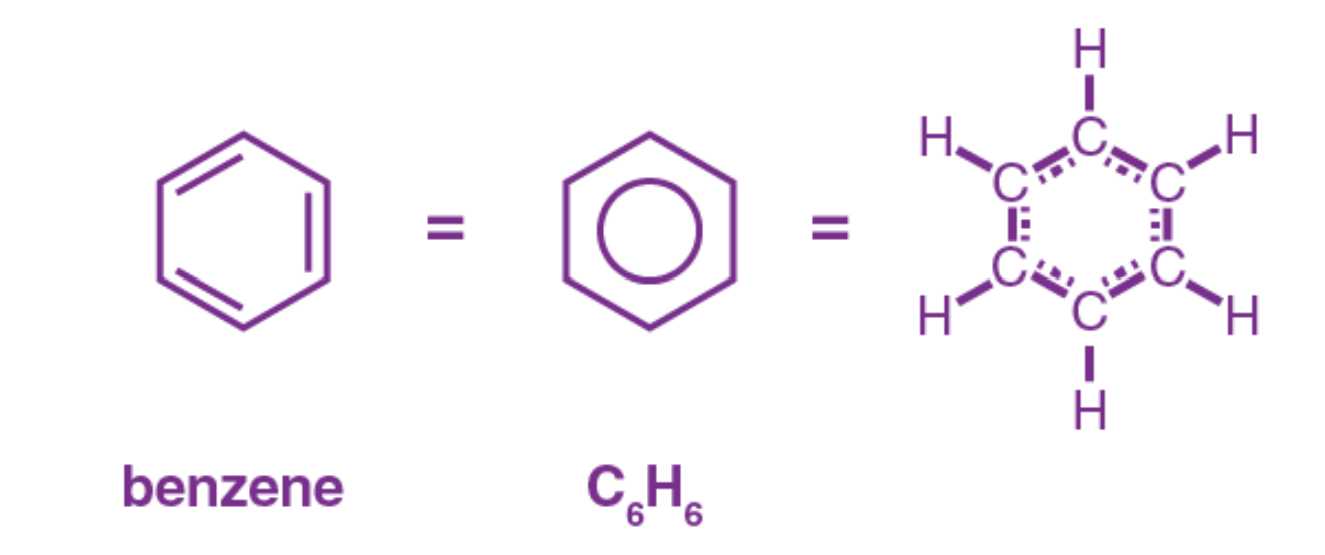
Aromatic Compounds
organic compounds that contain a ring of 6 carbon atoms with alternating double and single bonds
6 carbon rings referred to as “benzene ring”, “phenyl group”, or “aromatic moiety”
structural and kinky diagrams used often
often slightly volatile, fragrant, and commonly found in plant-derived molecules and synthetic pharmaceuticals
Benzene
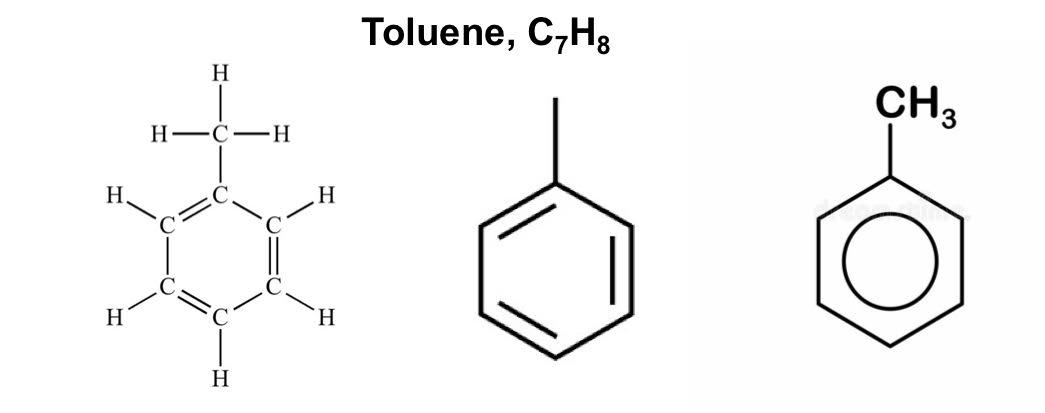
Toluene
C6H5CH3
Organic Contaminants
-Insecticides
-Herbicides
-Fungicides
-Rodenticides
-Organophosphates are less persistent and have largely replaced organochlorides
Volatile Organic Compounds
-generally only found in groundwater contaminants
-most common sources are leaking storage tanks and industrial discharges
Endocrine Disruptors
-compounds either known as hormones, mimic animal hormones, or otherwise interfere with the endocrine system
-can have detrimental effects on humans and wildlife even at trace concentrations
-”micropollutants” or PPCPs
-Sources of water contamination
Pharmaceuticals passed through urine, metabolites in urine
personal care products
Leaching from plastics
-Challenge: removal from wastewater is not cost effective
Poly- and Perfluoroalkyl Substances (PFAS)
-Carbon-fluorine bond is highly stable (544 kJ/mol)
-a large class of synthetic organic compounds wherein most or all alkyl carbon atoms are saturated with fluorine, rather than hydrogen
PFAS - Aqueous Film- forming Foams (AFFF)

Thermal Pollution

Equilibrium
Equilibrium chemistry - reversible
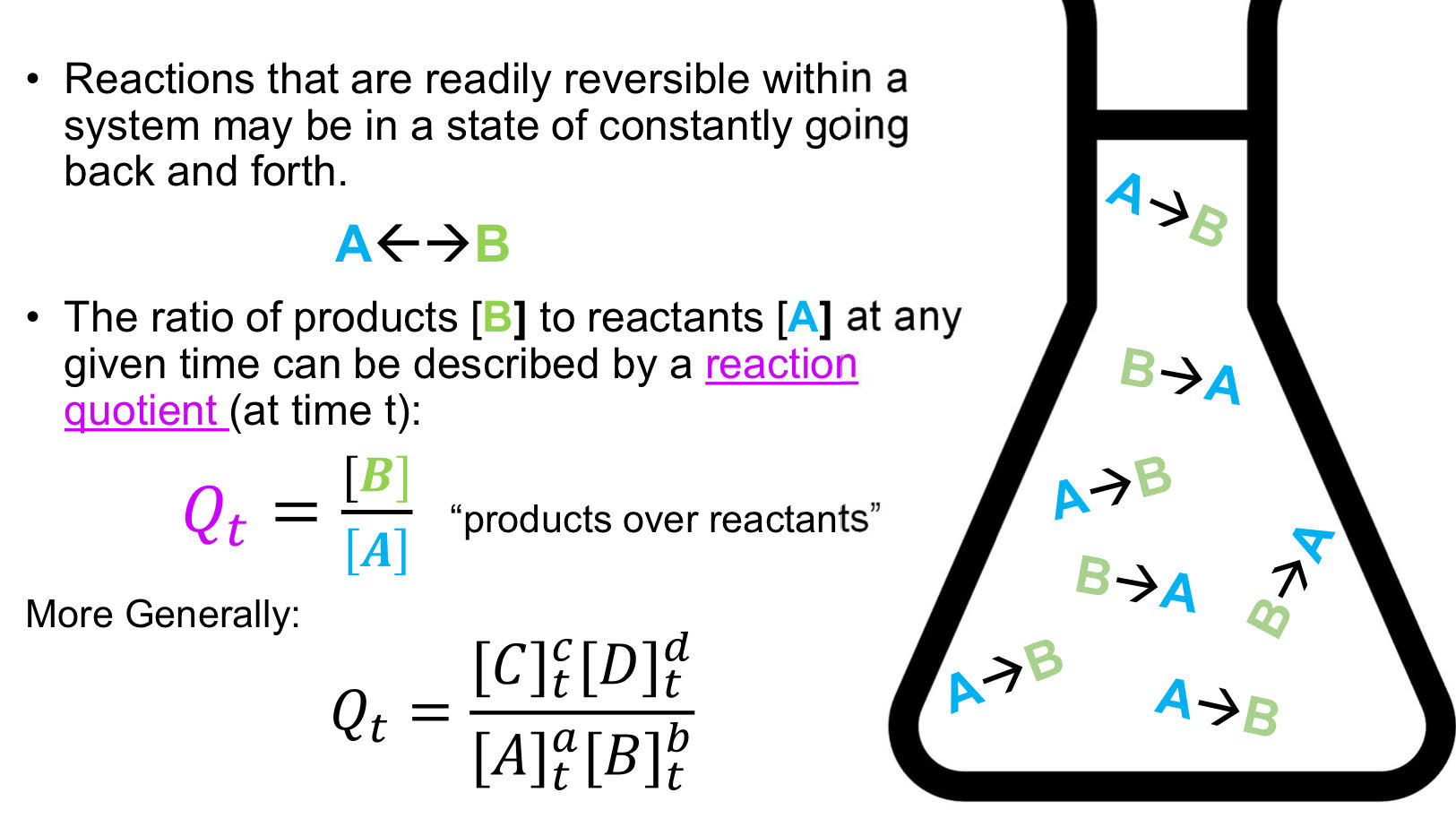
Chemical equilibrium
Under fixed conditions, such reactions will eventually reach a condition wherein the rate at which the “forward” reaction is proceeding is equal to the rate at which the “reverse” reaction is proceeding
At equilibrium: d[A]/dt = 0, d[B]/dt = 0
Equilibrium constant - K
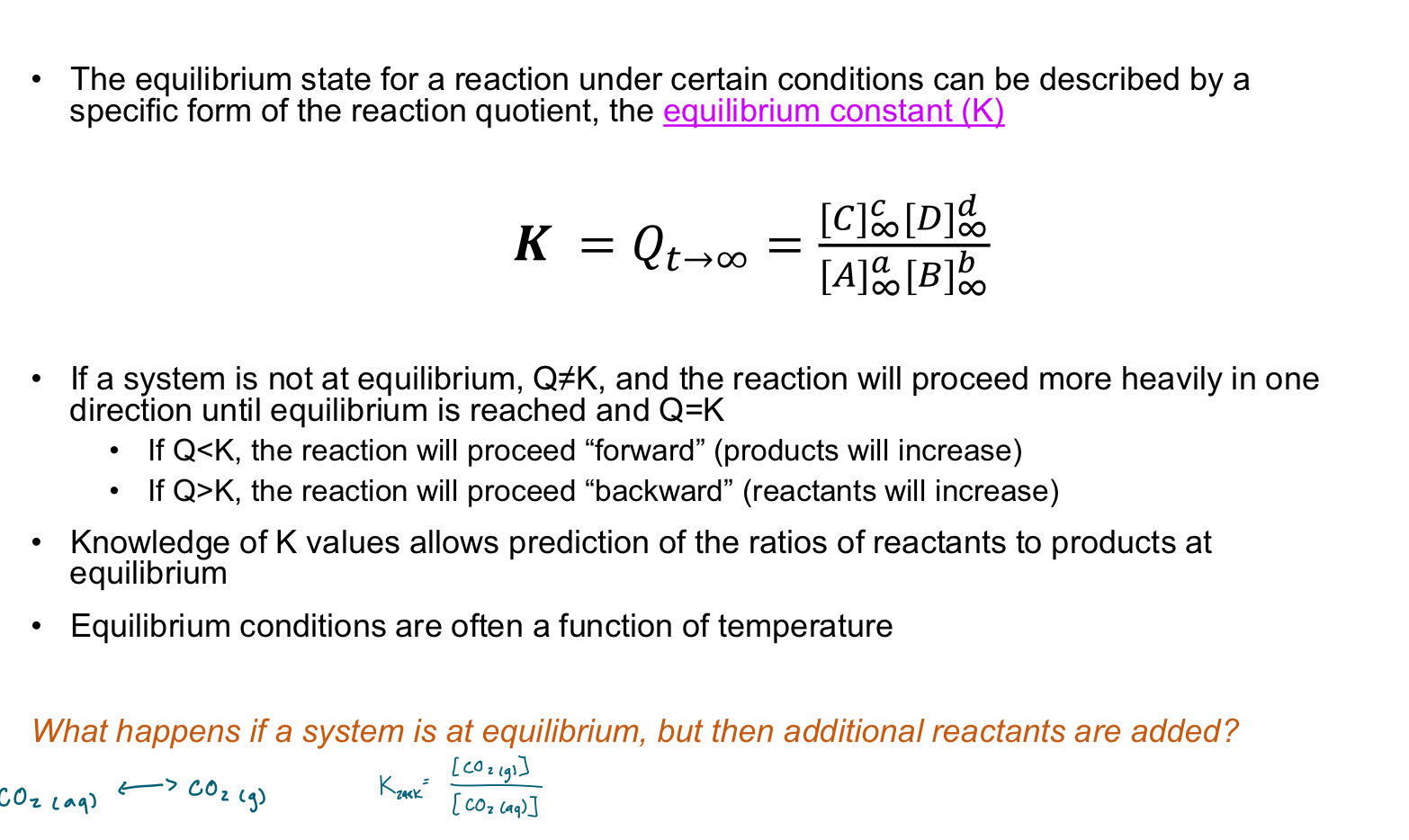
What influences the value of K?
-Thermodynamic preferences for certain phases, free energy associated with bonds forming.
-Lower temp encourages exothermic reactions and lower energy states
Acid dissociation constant, KA
The equilibrium constant for acid-base dissociation reactions
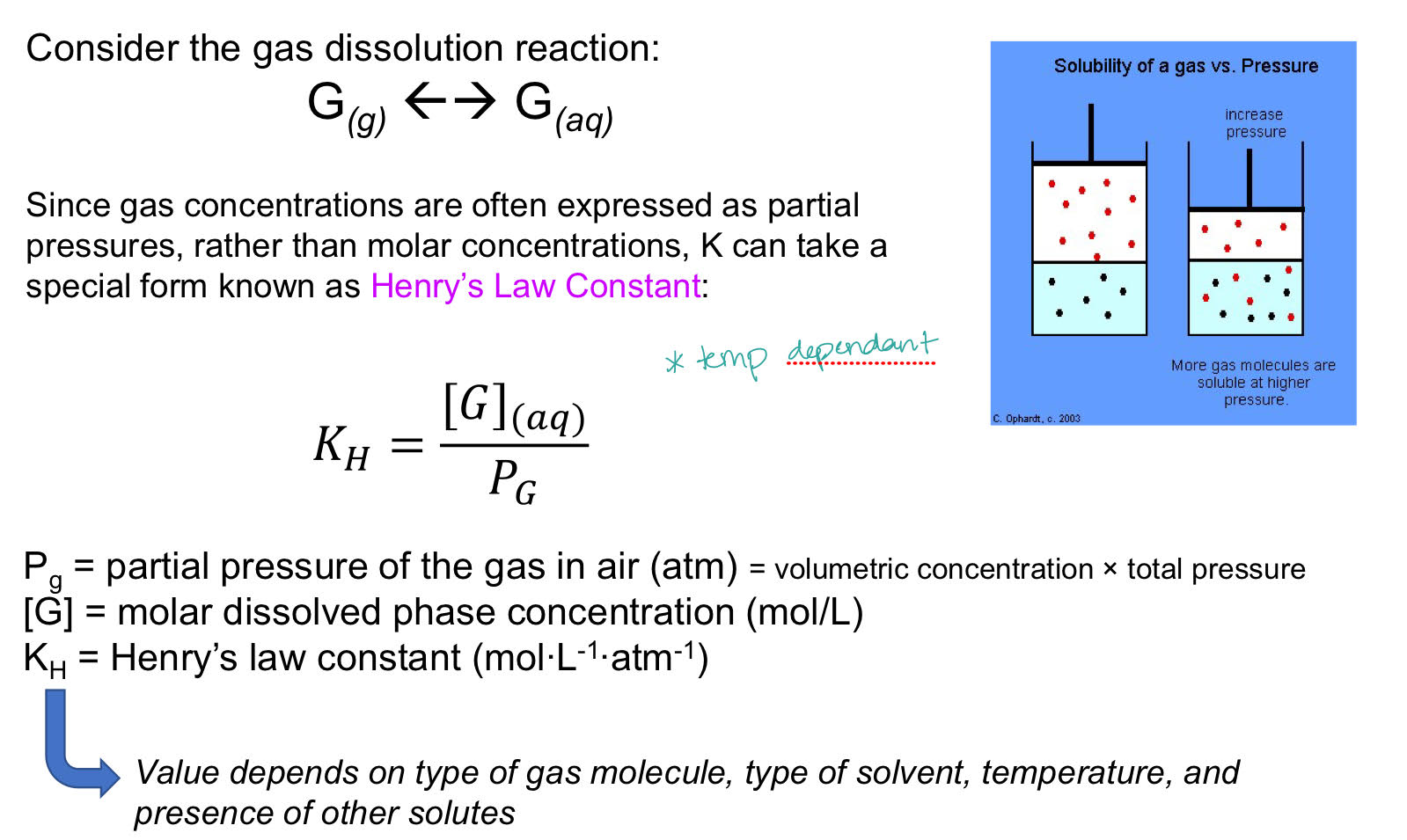
Gas-Liquid Equilibria
PV= nRT
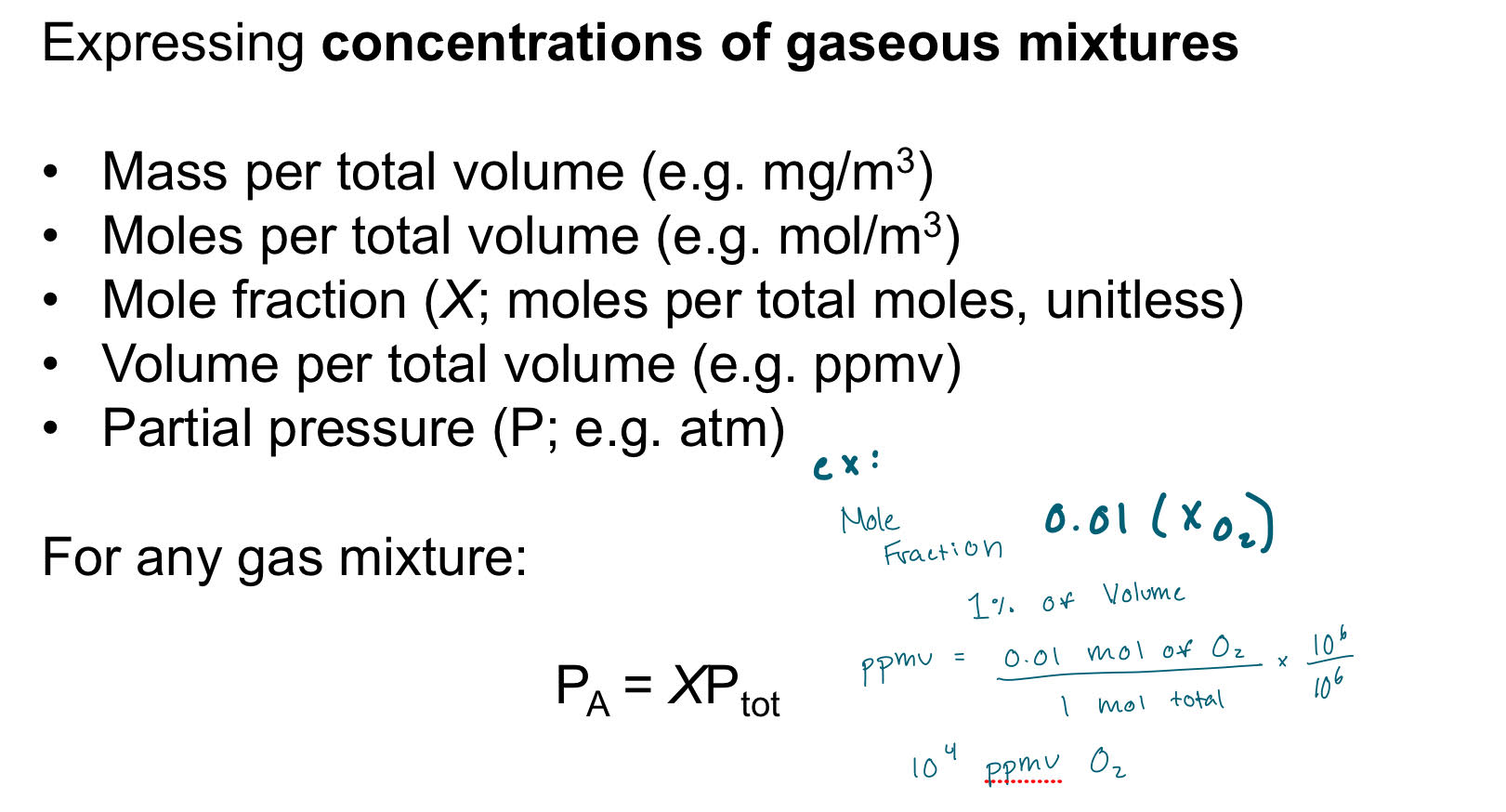
Henry’s Law
The Dissociation of Water

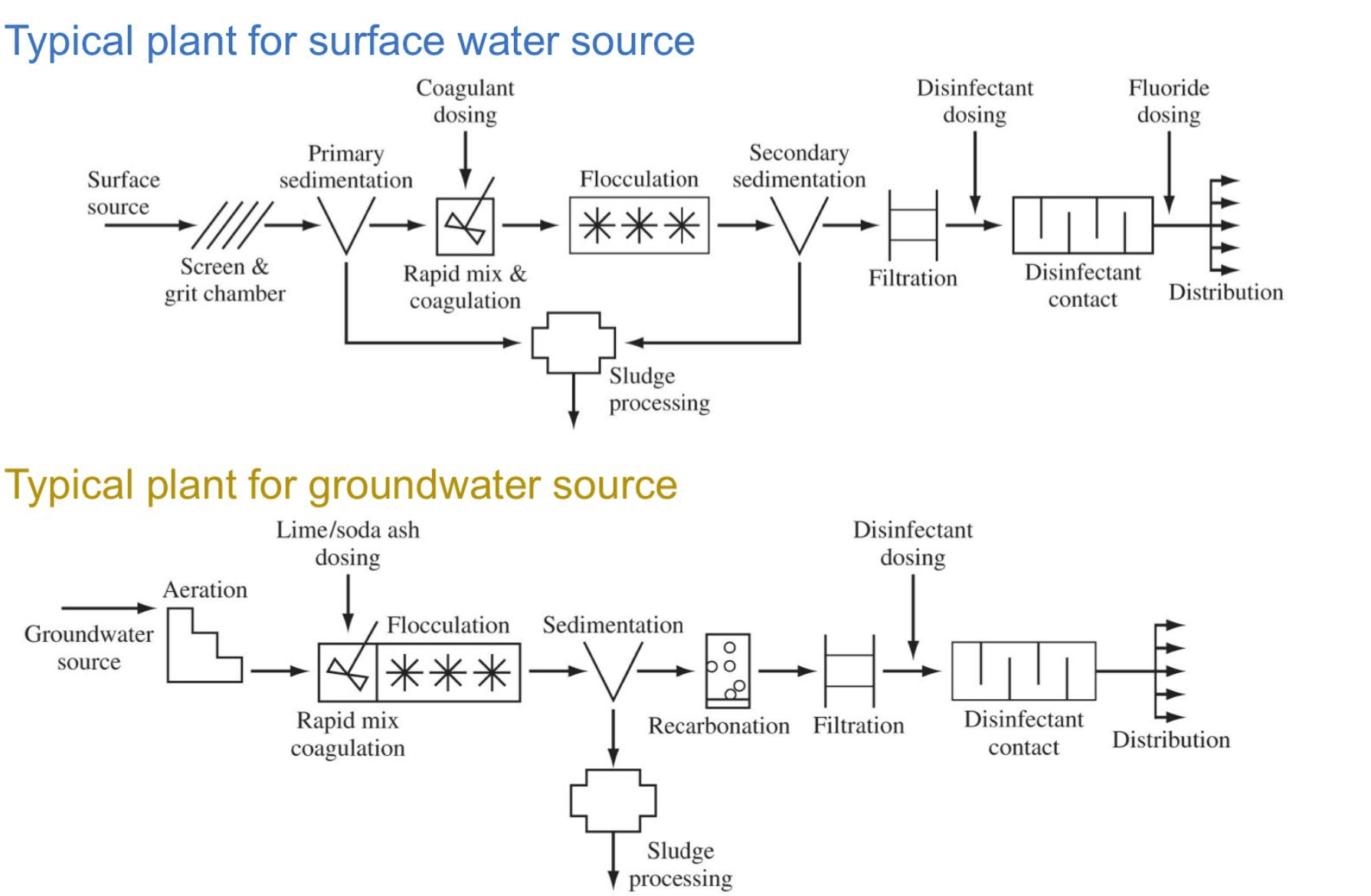
Municipal drinking water systems have three components:
-Source
-Water treatment plant
-Water distribution network
Drinking water sources
-Lakes and reservoirs
-Rivers
-Groundwater
-Seawater
Main goal of drinking water treatment plants
removal/inactivation of pathogens and provision of disinfectant residual
removal of harmful chemical contaminants
improve aesthetics of the water
reduce corrosiveness of the water
The United States Safe Drinking Water Act (1974)
-extended responsibility of the fed. gov. to regulate all community water systems with 25 or more customers
-establishes max. levels for both chemical and microbial contaminants. exceeding the limits can result in fines and legal penalties for municipalities
-uses Primary and Secondary Drinking Water Standards
SDWA- Primary Standards
-These are mandated thresholds for the purpose of protecting public health, come in two forms:
Maximum Contaminant Level (MCL) : states max. allowable conc. in treated water, typ. upon exit from treatment plant
Treatment Technique (TT) Standard : simply require a specific treatment practice to be employed
SDWA- Secondary Standards
-These are not required by the EPA, but are suggested limits on contaminants which pose more aesthetic or distribution problems. State agencies may make them mandatory
-They seek to address:
Taste and odor of water
Color
Corrosivity
Hardness (scaling of appliances and effect on washing/showering)
Fluoride for dental health
(Drinking) Water Treatment Systems
Source Water Quality Considerations
-The quality of water supply determines the necessary treatment steps employed in the treatment plant
-The most critical parameters are:
Turbidity/solids and microbial burden- Nephelometric turbidity units (NTU)
Hardness- removal is water softening
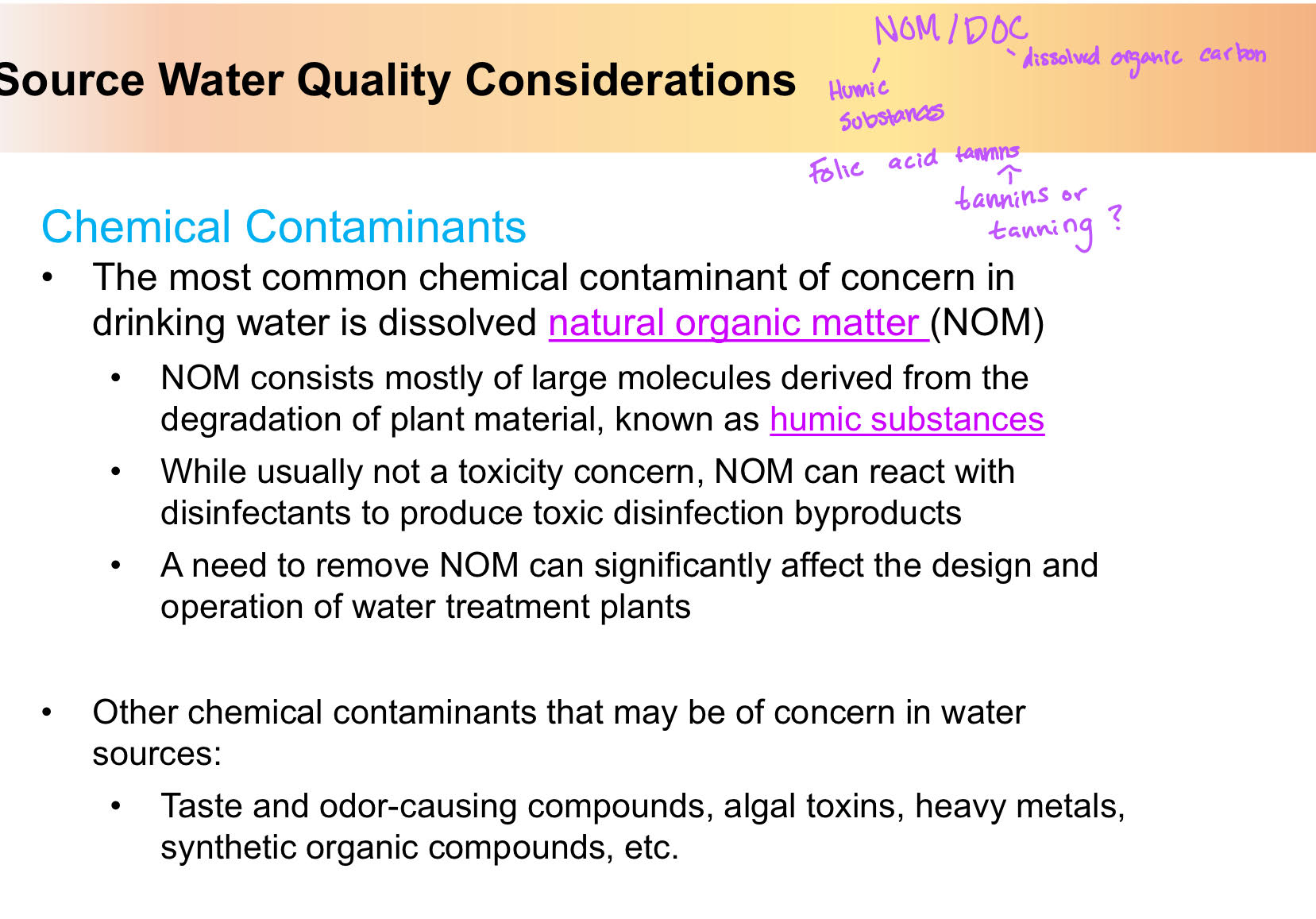
Organic carbon and chemical contaminants
pH and alkalinity
Chemical Contaminants
Source water quality considerations- pH and alkalinity

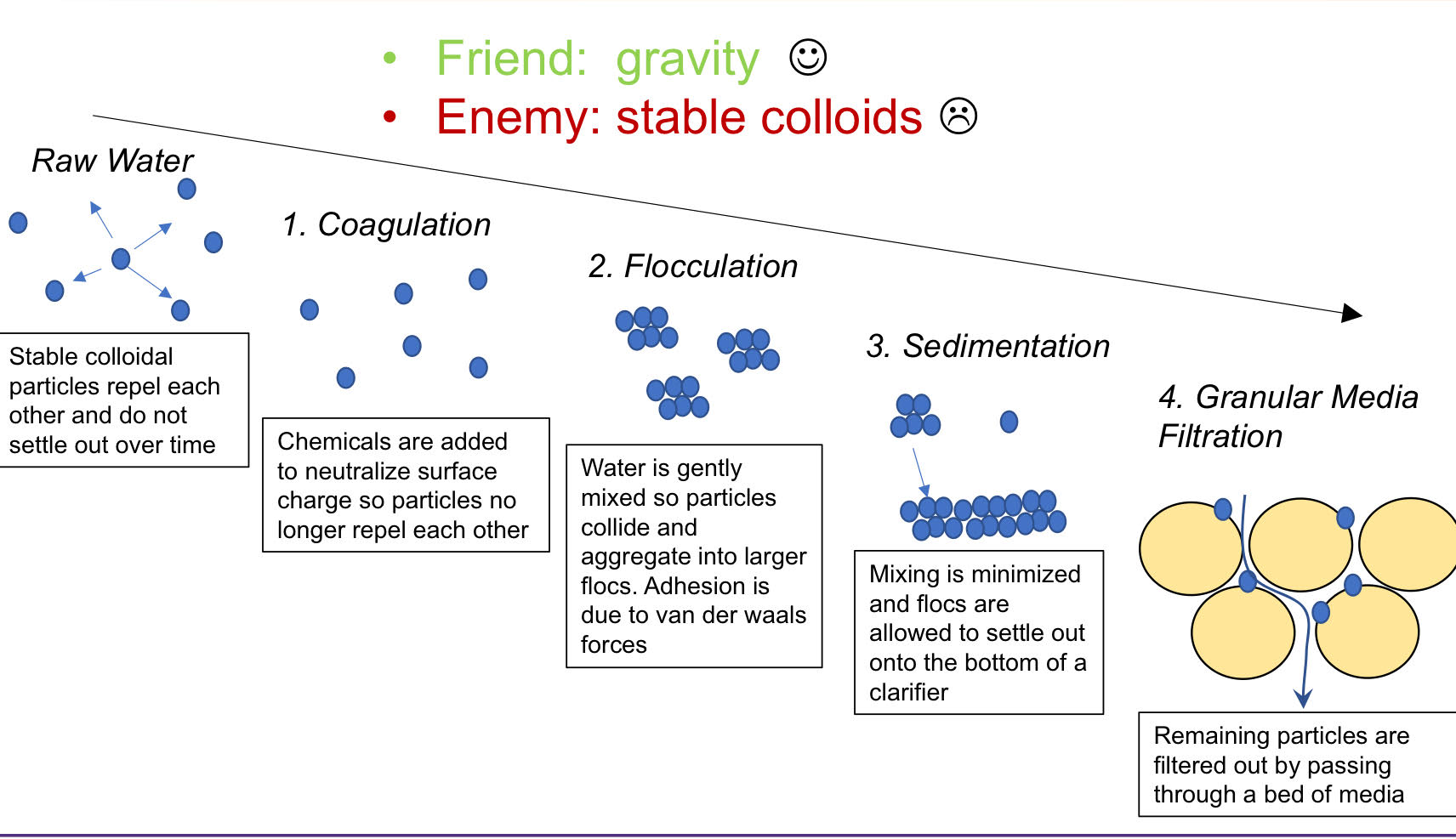
Solids Removal in Conventional Water Treatment: 4 Steps
Colloidal stability
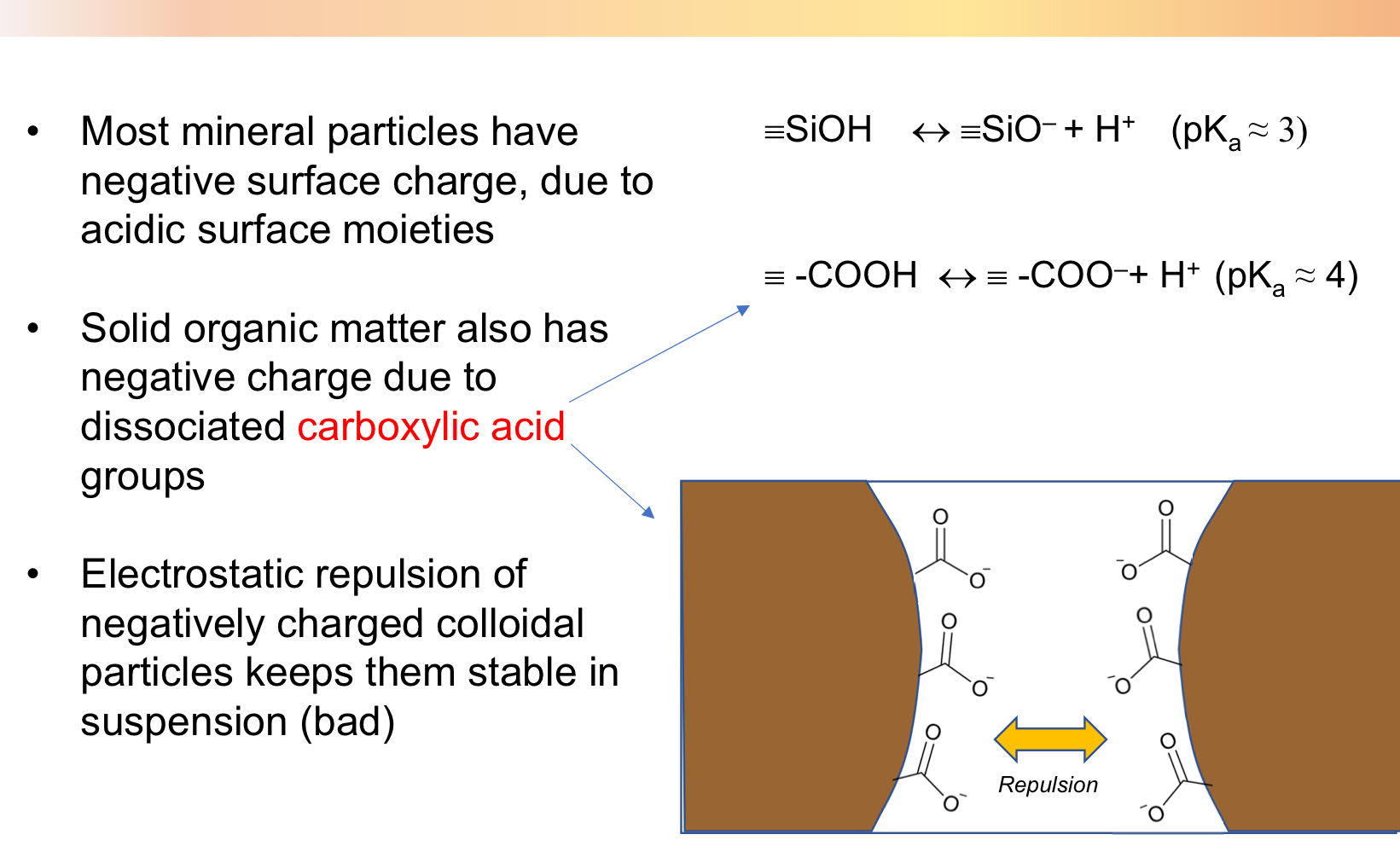
Coagulation
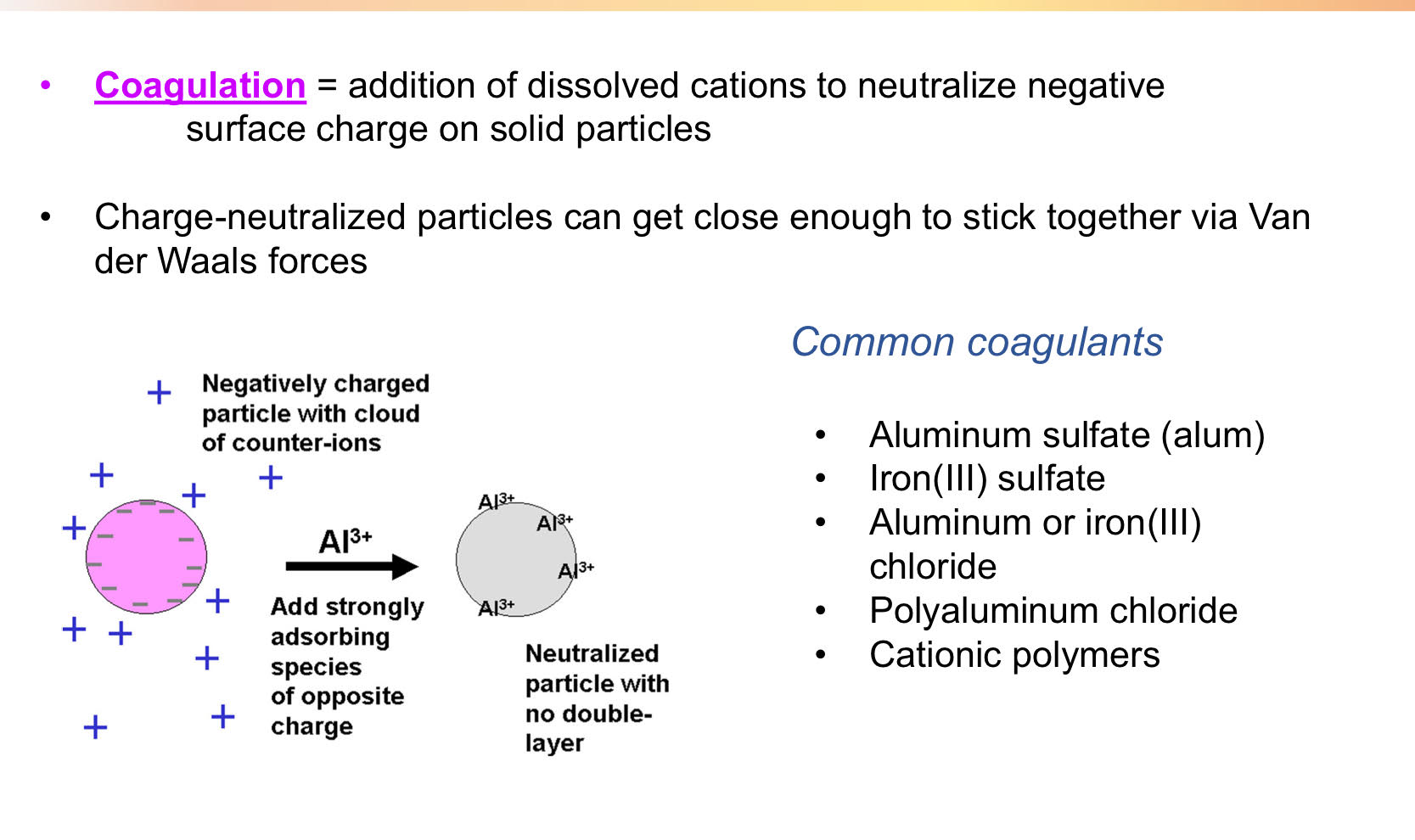
Flocculation
-Following addition of coagulants, water is mixed gently to induce flocculation- the formulation of loose aggregates of destabilized solids via mixing-induced collisions
Acid- Base Systems
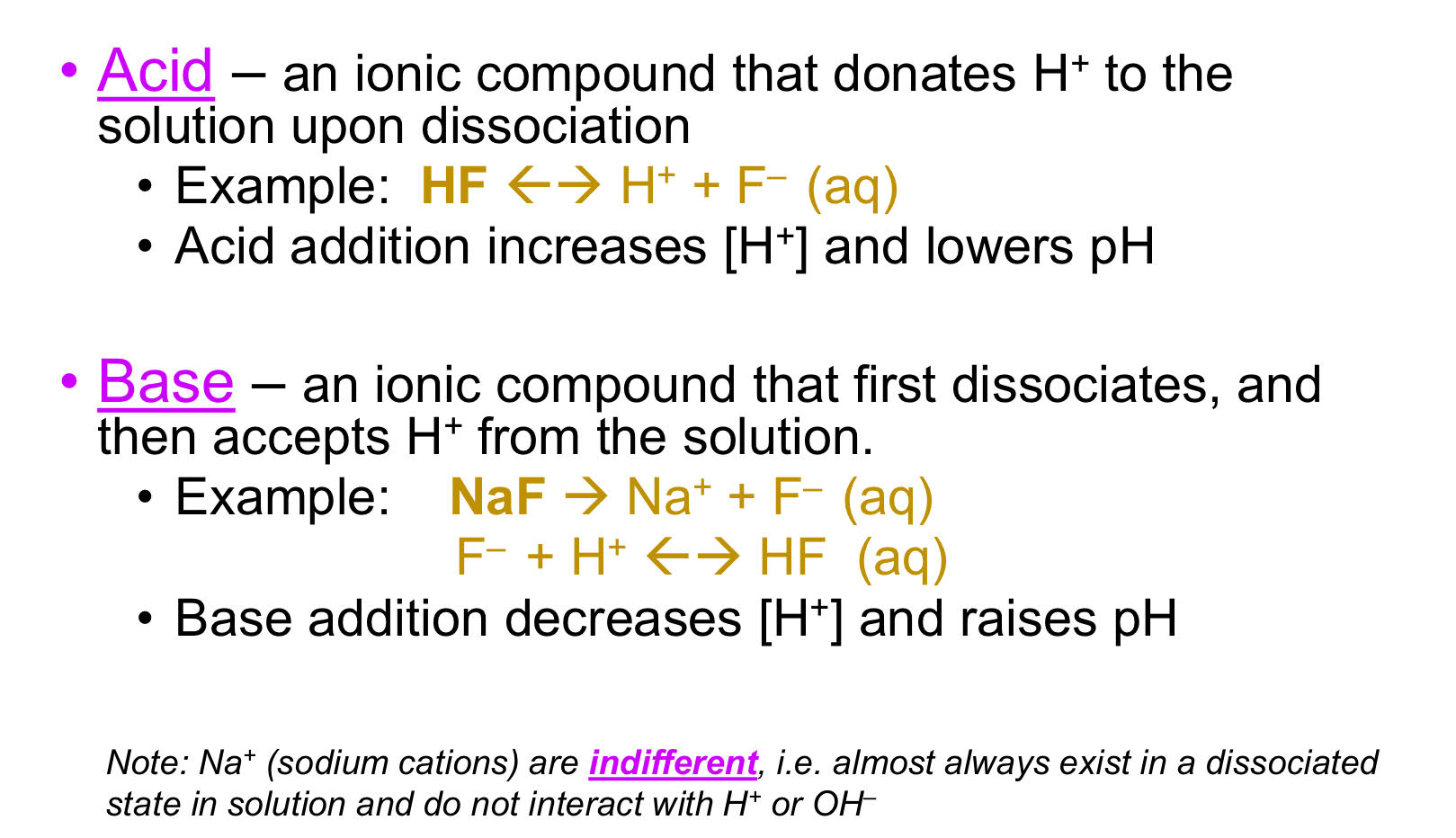
pH and pKA

Acid-base Systems: pH changes
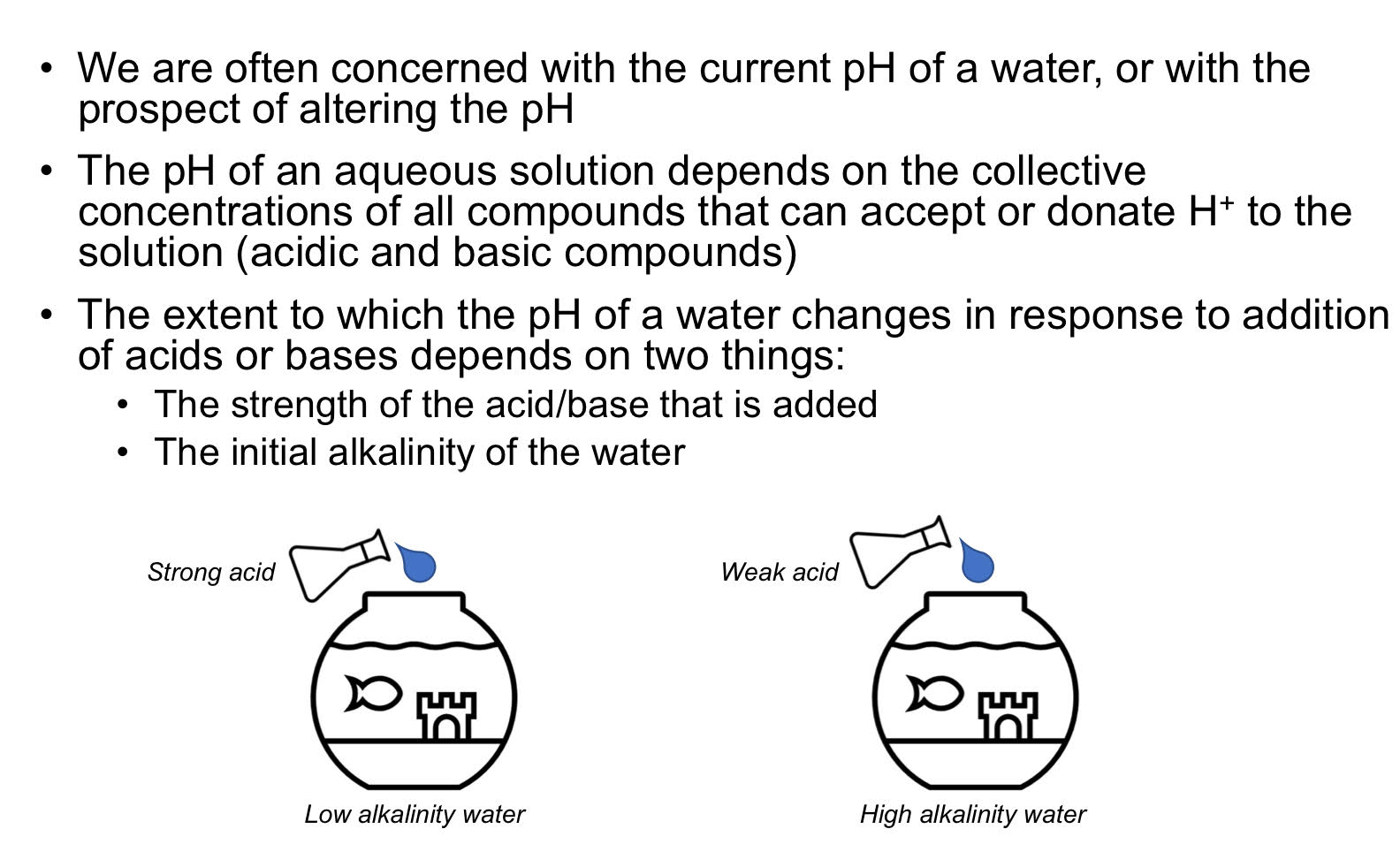
Acid-base Systems: Strong acids

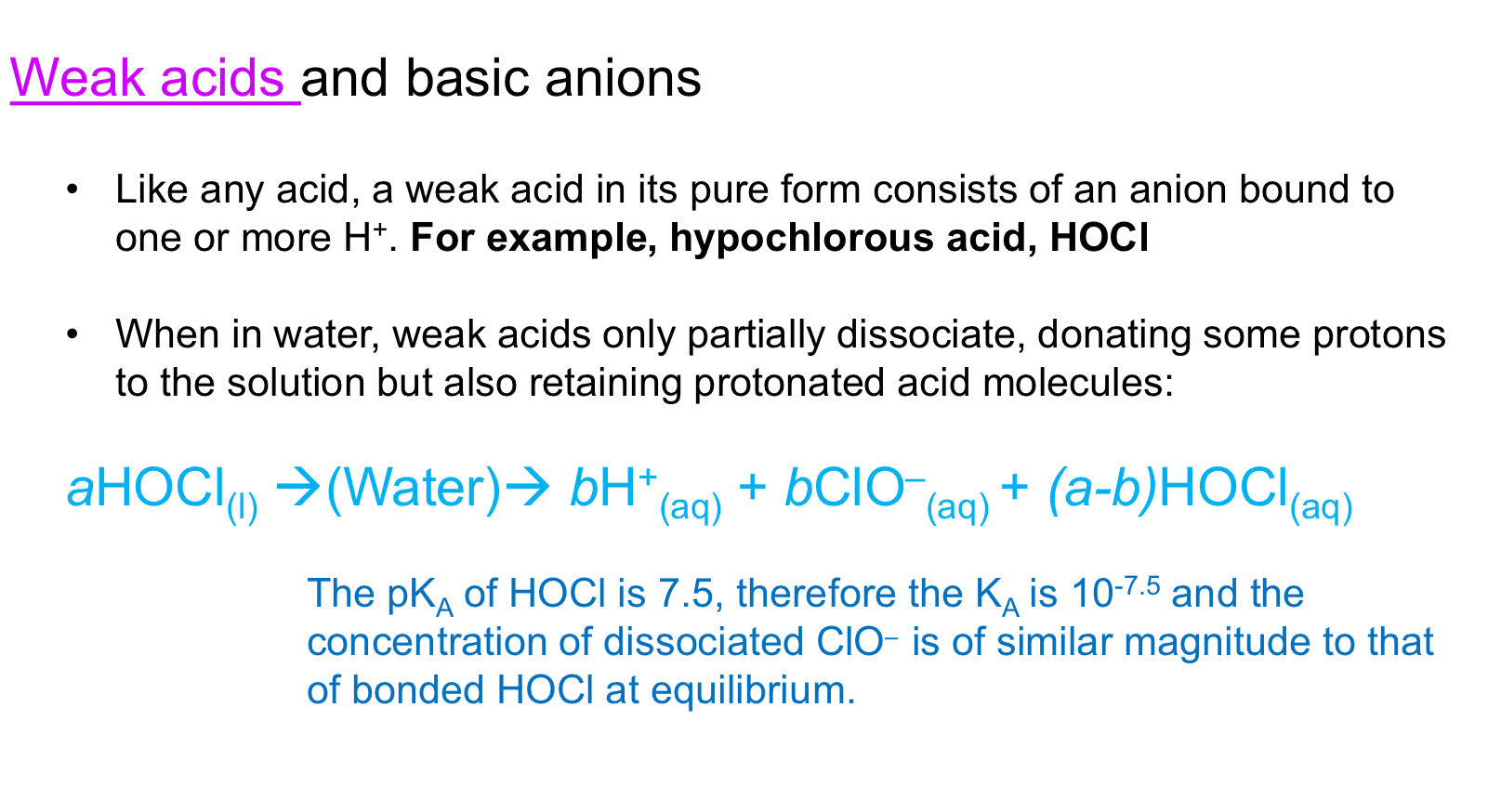
Acid-base Systems: Weak acids