Cyclic Compounds
1/24
Earn XP
Description and Tags
Last few lectures
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Describe the 3 types of strain that dictate the conformations of cycloalkanes
-Torsional strain, occurs in eclipsed molecules causing the bonds to get too close increasing the potential energy making it less stable/ more reactive.
-Steric strain, refers to the congestion caused by the physical presence of the surrounding ligands, which may slow down or prevent reactions at the atom.
-Angle strain, arises when the c-c-c bond angle in cycloalkanes is different from the normal tetrahedral bond angle (109.5), which is preferred for sp^3 hybridised carbon atoms. The further, away from 109.5 the less stable it is.
What’s the alternate name for angle strain
It’s also known as Baeyer strain
What does puckering of the ring refer too
Molecules can alter the amount of torsional and angle strain they experience by having molecules lay in different planes to each other.
What does butterfly conformation look like and what is the c-c-c bond angle
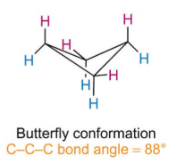
Describe how and why cyclobutane will form a butterfly conformation
When in the same plane there is lots of torsional strain as bond angle is 90.
To reduce torsional strain one of the four carbon atoms moves out of the plane and the ring forms a new shape.
This lower the angle slightly causing more angle strain but greatly decreases the torsional strain.
What does envelope conformation look like and what is the c-c-c bond angle
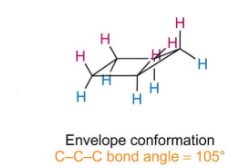
Describe why and how cyclopentane forms an envelope conformation
Cyclopentane has a bond angle of 108 but has lots of torsional strain due to having 1 pairs of eclipsed C-H bonds.
To minimise torsional strain, the ring puckers moving one of the carbons out the plane creating an envelope shape. This lowers torsional strain greatly but slightly decreases angle to 105.
Decrease in torsional strain outweighs the increase in angular strain, results in a more stable conformation.
What does chair conformation look like
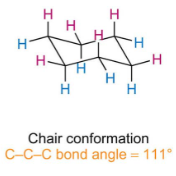
Describe why and how Cyclohexane forms a chair conformation
When planar the bond angle is 120 which is well above 109.5 giving great angular strain.
The ring puckers moving one carbon above the plane lowering both torsional straina and decreasing angle to 111 lowering angular strain.
When in chair formation there are two distinctive types of C-H bonds, describe them.
Axial bonds, when the C-H bonds will either point up or down vertically
Equatorial bonds, when the C-H bonds point outwards and are parallel to two of the C-C bonds in the ring.
Draw out a six axial hydrocarbon
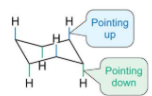
Draw out a six equatorial hydrocarbon
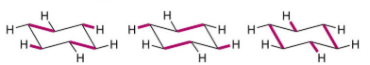
Describe the rotation about a single c-c bond in cyclic molecules
Due to being interconnected, there is no rotation about a single c-c bond
Briefly describe what a ring inversion is
Despite being no rotation about a single bond the entire molecule can rotate together.
The carbon atoms within the ring can move together leading to the conversion of one chair conformation into another chair conformation. This process of interconverting chair conformations is called a ring-flip or ring inversion.
(Does not only refer to chair conformations but most commonly will refer to them)
Describe how a ring inversion happens
When undergoing a ring inversion three atoms move up and the other three move down.
The change in position of the carbons results in all axial hydrogens becoming equatorial and vice versa.
What is the energy barrier for a ring flip and what rate does it do so
The energy barrier for a ring flip is 45 kJ mol^-1 so it will rapidly interconvert at RTP. Flips at a rate of 1x10^5 times per second.
Describe why it is impossible to distinguish between equatorial and axial hydrogen atoms
The energy barrier to rotation is very low and the speed the ring flips is very fast
Due to this rapid change the axial and equatorial hydrogens are equivalent when averaged over time, so it means it is impossible to actually distinguish between equatorial and axial hydrogen atoms .

What are the three most important intermediates of the ring inversion in Cyclohexane

How does a cyclohexane being substituted effect its conformations
Cyclohexane normally has equivalent chair conformations, but if a group other than hydrogen is bonded to the ring, then the two conformations are no longer equivalent.
Which position is the least stable
Generally, chair conformations with the substituent in an axial position has the higher energy so is less stable.
Why is axial less stable then equatorial
Due to having both more steric strain and torsional strain.
The substituent groups are close to the axial hydrogens at carbons 3' and 5' , which are parallel to the substituent and on the same side of the ring. Leads to steric strain, which increases the energy of the conformation.
What’s the name for the steric interactions in the axial position
1,3-diaxial interactions, due to 3 carbon atoms separate the axial substituents and the axial hydrogens it interacts with.
How does the size of substituents effect 1,3-diaxial interactions
Larger substituents are closer to the 1,3-diaxial hydrogens meaning their will be stronger interactions making the molecule less stable.
How is the stability of cycloalkanes determined
torsional strain, steric strain and angle strain
Describe trends stability of cyclic compounds
-cyclopropane and cyclobutane are more highly strained than other cycloalkanes. This is predominantly due to angle strain in the 3- and 4-membered rings.
-As the size of the cycloalkane increases, so the angle strain reduces and cyclopentane has low strain and cyclohexane has no strain. This explains why 5- and 6-membered cycloalkane rings are the most stable and the easiest to form.
-Cycloalkanes containing seven carbons or more are slightly less stable than cyclohexane (due to increased torsional strain and also steric strain caused by the interaction of hydrogen atoms across the ring), but after nine carbons the rings become more stable.
-Large-ring cycloalkanes have almost strain-free conformations because the long chains can adopt a structure that is very similar to that of straight-chain alkanes.