cell bio exam 3: membranes + transport
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What is lateral diffusion?
rapid, horizontal movement of lipids and proteins within a leaflet/monolayer
What factors regulate membrane bilayer fluidity?
chain length:
longer chain = more rigid
shorter chian = more fluid
saturated vs. unsaturated:
saturated = straight, packed, more rigid
unsaturated = kink/bend, more fluid
temperature:
cold = more rigid
warm = more fluid
cholesterol
too rigid → breaks up tight/packed interactions → introduces fluidity
too fluid → restricts movement of fatty acids —> introduces rigidity/integrity
How do phospholipids move
lateral: flexion, rotation
transverse: flip-flop (rarely occurs)
what are the three categories of membrane proteins? how do they interact with membrane bilayer?
integral: spans entire membrane bilayer; strong hydrophobic interactions with hydrophic tails; often alpha helical or beta barrel structures
peripheral: loosely attached to membrane surface via. hydrophilic interactions w/ hydrophilic polar heads
lipid-anchored: post-translational modification → covalently attached lipid that anchors protein onto membrane
How is membrane fluidity measured?
FRAP: fluorescence recovery after photobleaching
outer leaflet is fluorescently labeled
bleach area with laser
monitor recovery of fluorescence in the bleached area
results
fast recovery: high lateral mobility and membrane fluidity
slow/no recovery: restricted mobility, possibly mobility
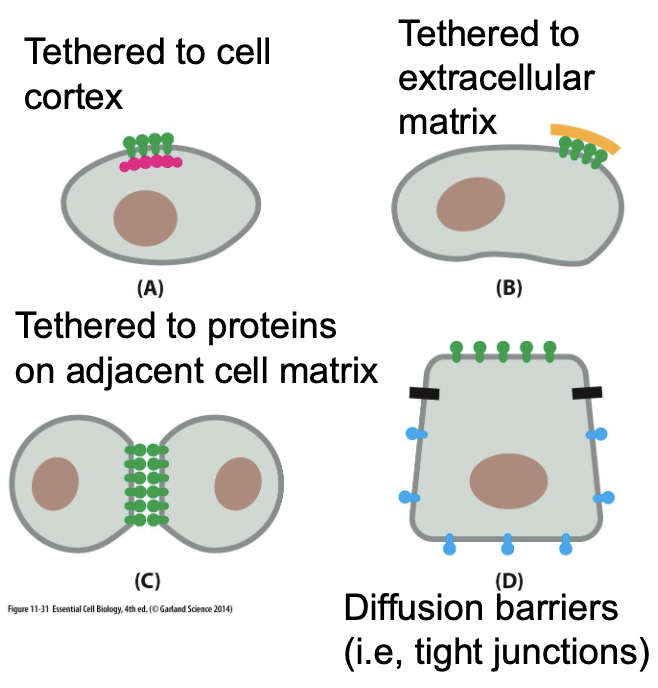
What mechanisms restrict protein localization within membranes?
tethered to cell cortex — proteins anchored to cytoskeleton
tethered to extracellular matrix
tethered to proteins on adjacent cell matrix (cell-cell adhesion)
diffusion barriers (tight junctions) — restrict lateral diffusion of proteins and lipids; segregates between apical and basolateral membrane domains
What is glycocalyx?
sugar coating that reinforces plasma membrane
always on extracellular leaflet on plasma membrane?
What are glycoproteins?
transmembrane protein w/ short, branched chain of sugars covalently attached to surface of plasma membrane
function: communication and recognition
What are proteoglycans?
transmembrane protein with long, unbranched polysaccharide chain
function: structural and signaling functions in the extracellular matrix
What is cell cortex?
framework of cytoskeletal proteins attached to plasma membrane
function: structural support, cell shape, anchors transmembrane proteins tethered to cortex,
What are tight junctions?
formed by claudin protein
forms a barrier between adjacent cells to control the movement of
prevents movement of proteins and lipids between apical and basolateral sides to maintain cell polarity
found in epithelial and endothelial tissues
what is apical-basal polarity?
apical: faces external environment (gut cavity)
basolateral: faces interior environment and bloodstream
what is passive transport?
transport with concentration gradient (high to low conc)
no energy required
what is simple diffusion?
passive transport
small, uncharged (nonpolar + polar) molecules pass freely through plasma membrane
e.g., O2, CO2, H2O
no transporter required
what is facilitated diffusion?
passive transport through channels or transporters
no energy required
what is channel-mediated transport?
transport of selective ions down concentration gradient (passive transport)
channel either open or closed conformation
fast movement of LOTS of ions
what is transporter-mediated transport?
SLOW transport — moves one or few molecules at a time
when open to outside, closed to inside
passive transport
what is active transport?
movement of molecules against concentration gradient (low to high conc.)
requires energy
uses pumps to transport ions/molecules
What are different types of active transporters?
symporter: both molecules move same direction and against their conc. gradient
antiporter: molecules move in diff directions and against their conc. gradient
switches between two conformational states ONLY if one binding site is occupied
does not switch conformational states if BOTH/NO binding sites are occupied
**both are secondary active transport
what is the sodium-potassium pump?
use ATP to move Na+ and K+ against their concentration gradient
3 Na+ out, 2 K+ in
what is sodium-proton exchanger (NHE)?
exchanges one intracellular H⁺ (proton) for one extracellular Na⁺.
antiporter
maintains neutral intracellular pH by removing excess H+ in cytosol
What is GLUT?
uniporter that facilitates diffusion of glucose down its concentration gradient
located at basolateral membrane of intestinal epithelial
What is SGLT?
sodium-glucose symporter
couples glucose uptake against its gradient with Na+ moving down its conc. gradient
located at apical membrane
glucose and sodium move from intestinal lumen to epithelial cell
what are the different ways that ion channels can be gated?
ligand-gated: molecule binds to channel and induces conformational charge
mechanically-gated: protein-protein interaction forces channel open
voltage-gated: channel opens when there is a change in membrane potential
what is membrane potential?
difference in electrical charge between the surface of the outside and inside of the membrane
what is patch clamping?
measures movement of ions through ion channel (channel activity
change in current when channel is open
what is signal propagation?
opening of one ion channel increases positive charge of membrane potential
relays signal and stimulates opening of neighboring channel
how is an action potential triggered?
rapid depolarizing stimulus triggers change from -60 mV to -40mV (threshold potential)
when membrane reaches threshold potential, voltage-gated Na+ channel opens → Na+ rushed into cell and increases membrane potential
@ +40 mV repolarization occurs
voltage-gated Na+ channels inactivate → no more Na+ enters
voltage-gated K+ channels open → K+ leaves cell and membrane potential is lowered towards resting membrane potential
hyperpolarization
Na+ channels are closed but not locked
K+ channels are slow to close → extra K+ leaves
membrane potential briefly becomes more negative than resting potential (-80 mV)
Na⁺/K⁺ pump and leak channels gradually restore the resting potential
refractory period: no action potential will be stimulated during this time
what are the conditions of a cell at resting state?
resting membrane potential: -70 mV
Na+/K+ and leak channels maintain high K+ inside and high Na+ outside
voltage-gated Na+ and K+ channels are closed
how are neurotransmitters released?
action potential reaches presynaptic terminal and depolarization opens voltage-gated Ca2+ channels
Ca2+ rushes into presynaptic terminal → triggers neurotransmitter release
neurotransmitter-storing vesicles are docked at membrane; SNARE protein help position vesicle
vesicle membrane fuses with presynaptic membrane; neurotransmitters are released into synaptic cleft via. exocytosis
neurotransmitters diffuse across synaptic cleft and bind to receptors on postsynaptic membrane
What is Co-IP?
co-immunoprecipitation: observes protein interactions
1. cell lysis to release proteins w/o disrupting interactions
antibody bindsb to target protein (“bait”)
precipitation: antibody-protein complex pulled out of solution
wash to remove unbound protein
elute + detect what other proteins bound to “bait” were pulled out of solution
what is co-translational synthesis?
translation/protein synthesis begins in the cytosol, resumes in endoplasmic reticulum, and then processed + secreted
how is co-translational synthesis initiated?
ribosome translates mRNA in cytosol
SRP (signal recognition particle) binds to ribosome and signal sequence as it emerges from ribosome (typically hydrophobic) → pauses translation temporarily
SRP-ribosome complex binds to SRP receptor on ER membrane
SRP is released → peptide passed to protein translocator and protein is threaded across ER membrane via. channel in translocator
signal sequence stays in translocator, exits lateral gate, and cleaved by transmembrane signal peptidase
translation resumes and growing polypeptide is threaded into ER lumen or inserted into ER membrane
What is soluble ER protein? What is their fate?
soluble ER protein: protein that is freely floating in ER lumen
fate -
undergo folding with chaperones and post-translational modifications
may either stay in ER, packaged into vesicles + transported to organelles, or secreted from cell
what is a single-pass transmembrane protein?
goes through membrane ONCE
has ONE transmembrane domain
what is the orientation of a single-pass transmembrane protein w/ cleavable signal sequence?
N-terminus in lumen of ER
C-terminus faces cytosol
what is the fate of single pass transmembrane proteins with cleavable signal sequence?
as polypeptide is threaded into ER lumen, hydrophobic stop-transfer sequence enters translocator → stops further translocation of segment into ER lumen
hydrophobic segments laterally exits translocator
hydrophobic sequence pushes ribosome off ER membrane → translation resumes in cytoplasm ribosome
what happens to single pass transmembrane protein w/o signal sequence?
SRP recognizes transmembrane domain (hydrophobic segment) when it emerges from ribosome and inserts protein into membrane
hydrophobic region fully spans membrane
orientation depends on location of (+) charged amino acids
terminus with more (+) charged amino acids faces cytosol
bc electrostatic interactions with negatively charged cytosol
ribosome continues translating remaining portion of protein
how are multipass transmembrane proteins inserted?
FIRST signal sequence/transmembrane domain dictates orientation
subsequent transmembrane domains follow in/out protein
what is glycosylation?
post-translational modification where glucose is covalently attached
nonspecific glycosylation occurs in ER
functions
chaperones bind to specific sugar groups to help proteins fold properly
glycosylation in cytoplasm indicate misfolding → triggers ERAD
what happens when there are misfolded proteins (small-scale)?
*glycosylation in cytoplasm is a marker for misfolded protein → triggers ERAD (ER-associated degradation)
glycanase cleaves glycosyl group from protein
ubiquitinase post-translationally modifies protein by covalently attaching ubiquitin (marker for protein degradation)
proteasome degrades protein and recycles amino acids
what happens when there are misfolded proteins (large-scale)?
*misfolded protein binds to receptor on ER membrane → activates UPR (unfolded protein synthesis)
reduce protein synthesis → less new protein enter ER
increases production of chaperones and ERAD machinery → help refold/remove misfolded protein
trigger apoptosis if stress can’t be reused
how does vesicular transport work?
vesicle buds off from donor compartment (e.g. ER, golgi)
vesicle moves through cytoplasm
vesicle fuses with target compartment and delivers cargo (e..g proteins, lipids)
what are coat proteins?
proteins that bind to transmembrane protein to bend ER membrane into curve
accumulation of coat protein causes vesicle budding
what is the difference between the two types of coat proteins?
COPII: moves vesicle from ER → golgi
COPI: moves vesicle from golgi → ER (retrograde transport)
why is COPII nonspecific?
ALL proteins found in ER are transported to golgi for processing
what happens if COPII is inhibited?
protein is stuck in ER
what are examples of proteins that would be retrogradely transported?
chaperones
signal receptor particle (SRP receptor)
translocator
signal peptidase
What is KDEL?
ER retrieval/retention signal that signals proteins are destined to stay in ER
short amino acid sequece (Lys-Asp-Glu-Leu) at the C-terminus
KDEL binds to KDEL receptor on golgi to induce conformational change that allows COPI to bind; accumulation of COPI induces membrane bending and vesicle budding
what happens if KDEL is inhibited?
KDEL ER protein is stuck in golgi
plasma membrane protein is secreted with no problem
How does fusing work?
*after budding, uncoating (removal of coat protein from vesicle) occurs, exposing surface proteins (e.g. v-SNAres, Rab-GTPases)
Rab GTPase binds to tethering proteins on target membrane → pulls vesicle towards target
every target membrane has different tether proteins
every rab binds to specific tether
docking — v-SNARE (vesicle) binds to t-SNARE (target) → zipping pulls membrane extremely close together
hemifusion — outer leaflets merger, inner leafletes remain separate
full fusion — inner leaflets fuse to form fusion pore → vesicle content is delivered to target and vesicle membrane is incorporated into target membrane
SNARE complex is disassembled by NSF and SNAP
How does patch clamp works?
use fine pipette to isolate a part of membrane and measure change in action potentials within the region
What is CTFR mutation? How can a drug resolve this?
mutation causes protein misfolding and prevents from reaching cell surface
Cl- channel is defective → Cl- can’t enter the cell
results in thick mucus
resolution
help CFTR deltaF508 fold
increase UPR chaperone expression → helps with folding correctly
increase expression of other Cl- channels
How does cholesterol stiffen the membrane?
forms stable hydrophobic interactions between rigid fused ring structure of cholesterol and hydrophobic acyl tails of phospholipids
what mutations could explain accumulation of protein in vesicles?
mutation on Rab
mutation on v-SNARE
what mutations could explain accumulation of protein in golgi?
mutation of COPI
mutation of ER retention signal on C-terminus
what does oubain do?