OA: Controlled Substances
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Controlled Substances
Drugs that have a high abuse potential.
Controlled Substance Act of 1970
Passed to reduce drug abuse.
Drug Enforcement Agency (DEA)
Regulates controlled substances.
DEA License
Required by all veterinarians that prescribe, order, or dispense controlled substances.
Locked Cabinet Requirement
Controlled substances must be kept in a locked cabinet that is irremovable from wall or ground.
Logging Requirement
Controlled substances must be logged.
Controlled Substance Schedule
Indicated by 'C' written on the bottle; five schedules exist.
Schedule I
Highest abuse potential, dispensed for research only; Restrictions: DEA form 222 required.
Schedule II
High abuse potential, dispensed via written prescription, no refills; Restrictions: DEA form 222 required.
Schedule III
Abuse potential less than schedule II, dispensed via written prescription, can refill five times; Restrictions: DEA number.
Schedule IV
Low abuse potential, dispensed via written prescription; can refill five times; Restrictions: DEA number.
Schedule V
Low abuse potential, no DEA limits; Restrictions: DEA number.
Form 222
A form required for ordering Schedule I and II controlled substances.
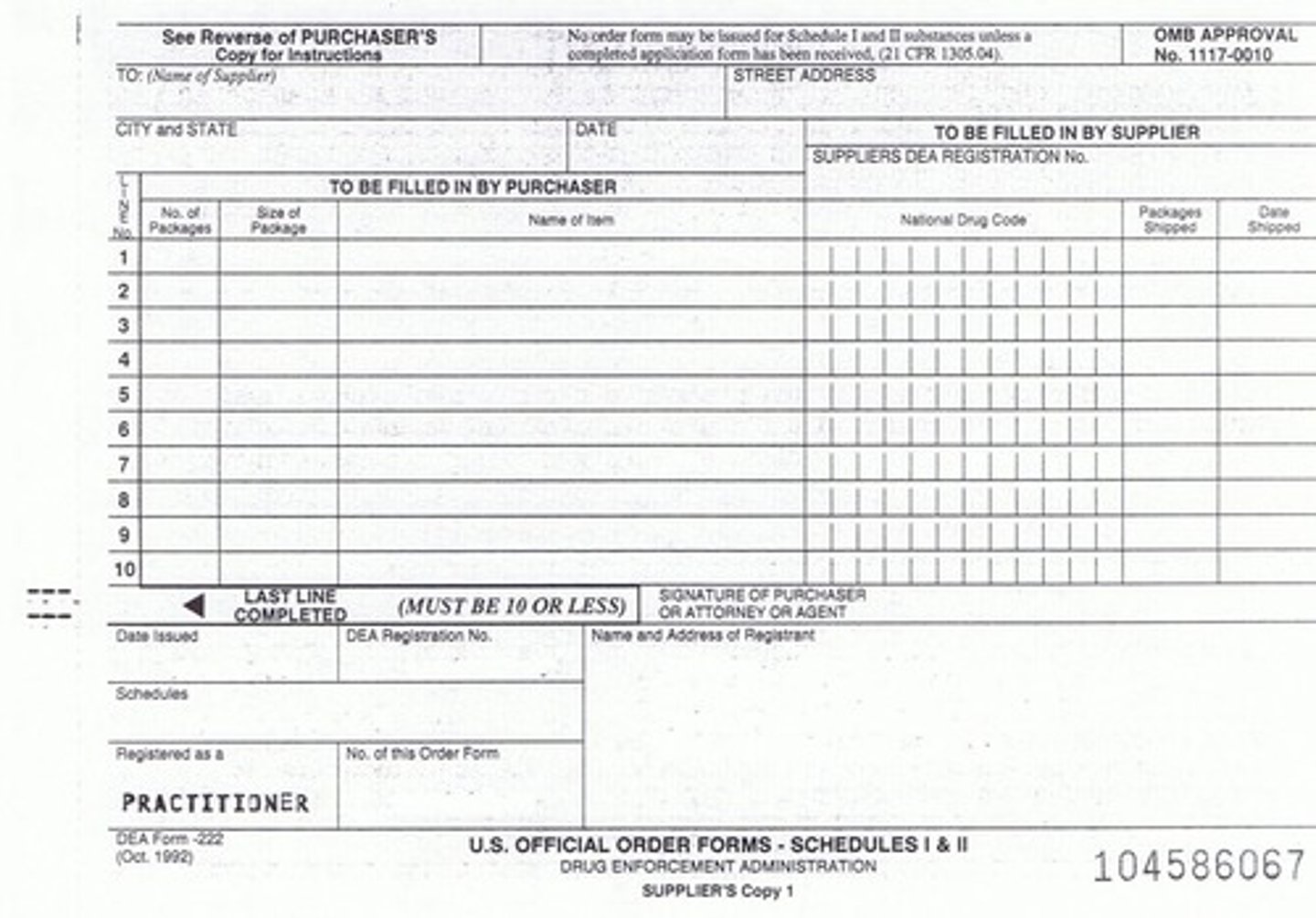
Veterinarian Registration
Veterinarians must register with DEA; some states require additional CS license.
Security and Protection
Includes reasonable hiring practices and limited access to controlled substances.
Safes for CS
Must be substantially constructed, anchored to wall or floor, used to store CS not in active use.
Daily Use Storage
Less substantially secured storage for daily use; cannot hold drugs while not in active use.
Record Keeping
CS records must be kept for 2 years; DEA can inspect at any time.
Separate Storage Requirement
Logs for Class II must be stored separate from Class III, IV, and V.
Inventory Requirement
Required when opening a business, biennial, and at the close of a business.
CS File Contents
Should include CS invoice, assigned bottle number, stock supply sheet, running drug log, drug discrepancies, and annual CS inventory.
DEA Form 106
Used to report CS loss of 3% or more.

Handling Expired CS
Send to a reverse distributor for incineration; fill out DEA Form 41 and maintain CS logs.
Annual Inventory
A required inventory of controlled substances conducted yearly.