Limitations of bonding diagrams and the particle model
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
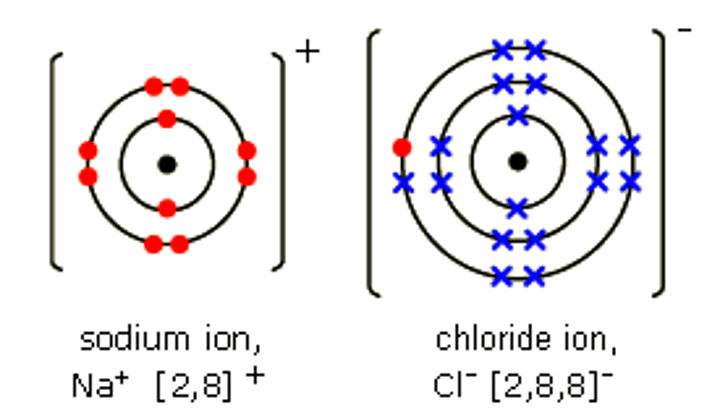
Pros/cons of dot and cross diagrams for giant ionic structures
Pro- we know exactly where electrons are coming from
Cons- don't show the structure or size of the ionic compound or how the ions are arranged.
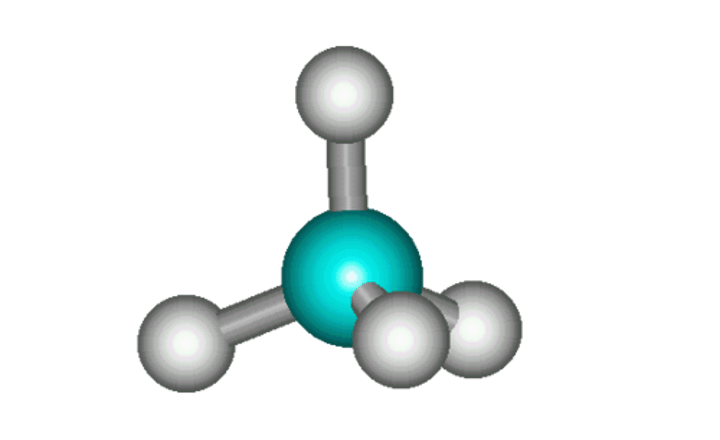
Pros and cons of ball and stick model for giant ionic structures
Pro- Can visualise the shape and arrangement of the molecule because
Cons
- Fails at indicating the movement of electrons
- The atoms are placed far apart from each other, which in reality is not the case as the gaps between atoms are much smaller
- Only show a portion of the structure, so cannot get an idea of the sie
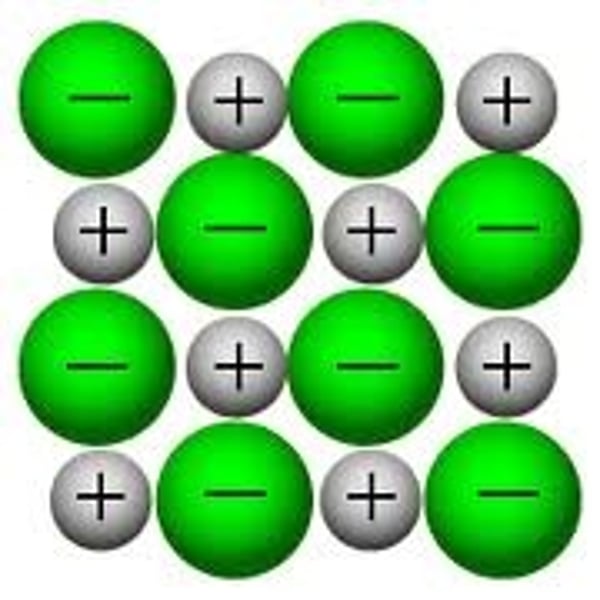
Cons of 2D model for giant ionic structures
- it does not show where the ions are located on the other layers.
- Cannot give you an idea of the shape of a molecule
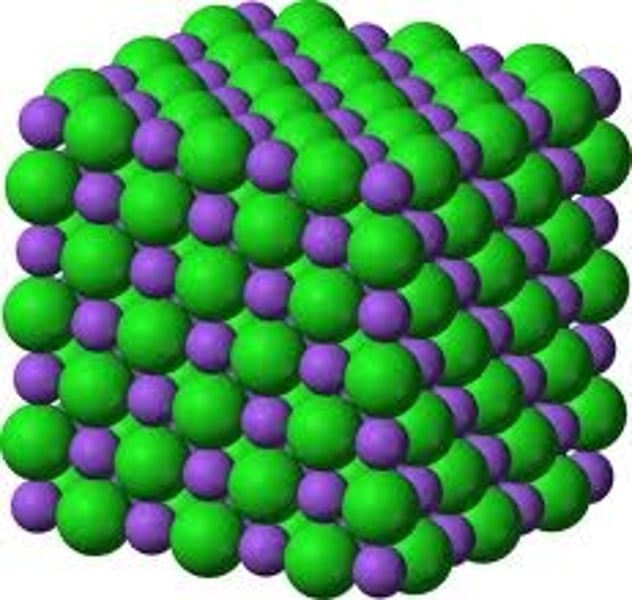
Pros and cons of 3D model for giant ionic structures
Advantages:3D drawings and models depict the arrangement in space of the ionsAlso show the repeating pattern in giant lattice structures
Disadvantages:Only illustrate the outermost layer of the compoundAre difficult and time-consuming to draw
Cons of 2d for covalent
- Doesn't give an idea of outer electrons that are not in bonds
- Can't tell which electron came from which atom
Limitations of particle model
- particles are represented by solid inelastic spheres, when actually they are different shapes
- assumes there are no forces between particles.
The amount of energy required for a change in state depends on
the strength of the forces between the particles
The stronger the forces between the particles...
- the more energy required to overcome these forces so
- the higher the melting point and boiling point of the substance