3.1.2 - Relative Formula Mass
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
Define relative atomic mass (RAM)
The average mass of atoms in an element taking into account masses and abundance of its isotopes, relative to 12C
2
New cards
Define relative formula mass (RFM)
The sum of RAM’s of all atoms in the formula
3
New cards
How do you calculate RFM?
Work out how many atoms of each element there are in the chemical formula.
Add together the Ar values for all the atoms of each element present.
4
New cards
What is the relative formula mass of:
CaF2
C6H12O6
CaF2 - (Ar values: Ca = 40, F = 19)
40 + 19 + 19 = 78
CHO - (A values: C = 12, H = 1, O = 16)
(12 × 6) + (1 × 12) + (16 × 6) = 180
5
New cards
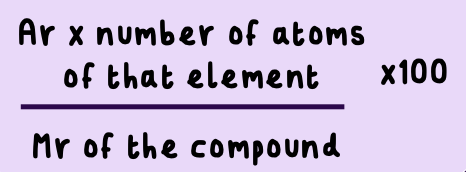
How do you find the percentage mass of an element in a compound?