MSE-
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
What are ceramics? How do they differ from metals?
ceramics are materials and nonmetallic compounds for which interatomic bonds are ionic or predominantly ionic with covalent bonds.
ceramics differ from metals in their properties in that they have a more complex crystal structure, have higher melting points, and aren’t electrical conductors
Name a few distinctive characteristics of typical ceramics
-high melting point
-hard & brittle as a result of ionic and covalent compounds
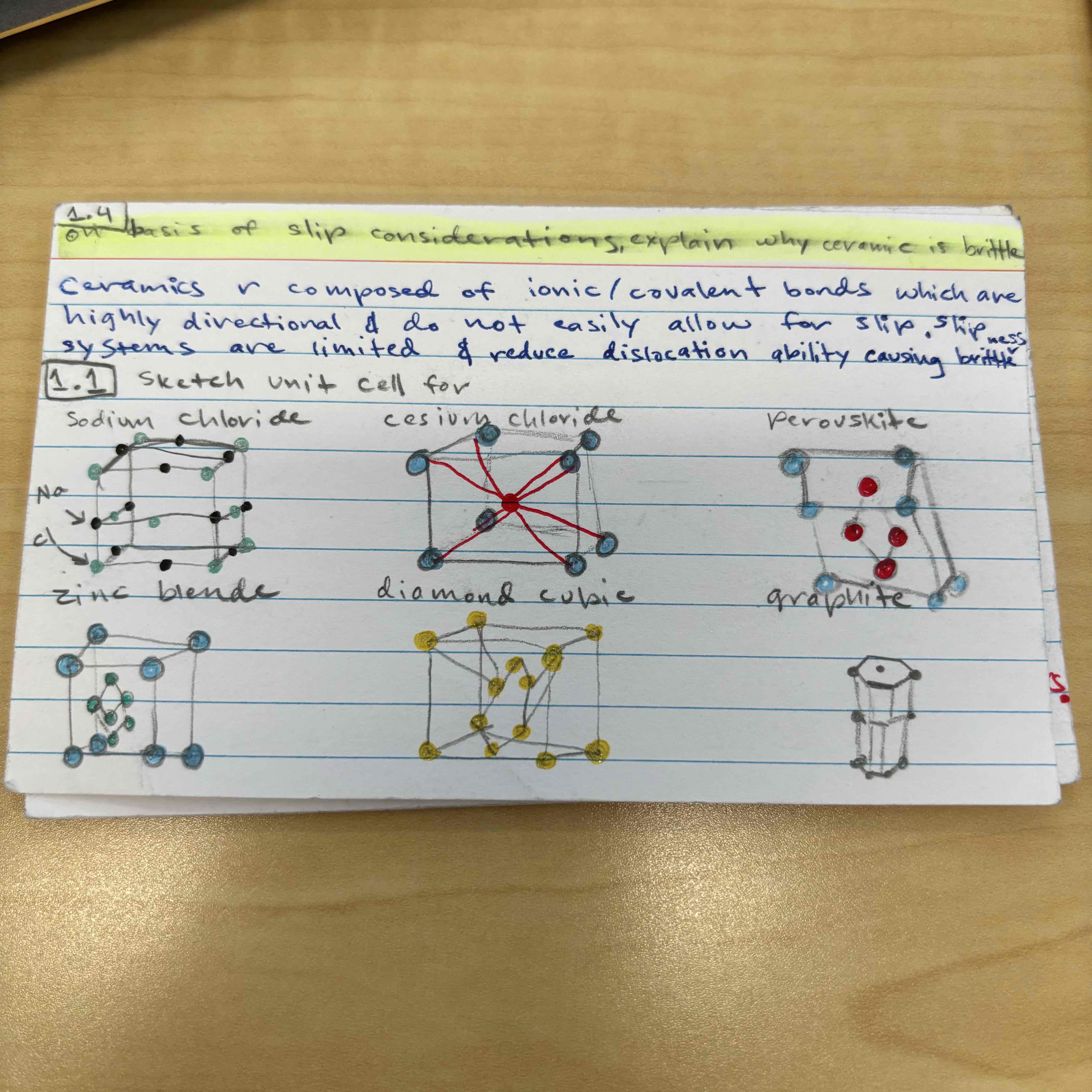
Name 4 different crystal structures of ceramics
-AX (rock salt)
-AX2 (fluorite)
-ABX3 (perovskite)
-AB2X4 (spinel)
whats an anion and a cation?
an anion is a negatively charged ion, a cation is a positively charged ion
whats a coordination # in a ceramic?
a coordination # is the number of anion nearest neighbors to cation
two defects in ceramics
frenkel (cation-vacancy x cation interstital)
schottky (cation vacancy x anion vacancy)
Sketch unit cell for:
what is silica & silicate
silica is the simplest material made up of silicon and oxygen
silicate is salts containing both silicon and oxygen
3 examples of ceramic materials
sand
clay
zirconia
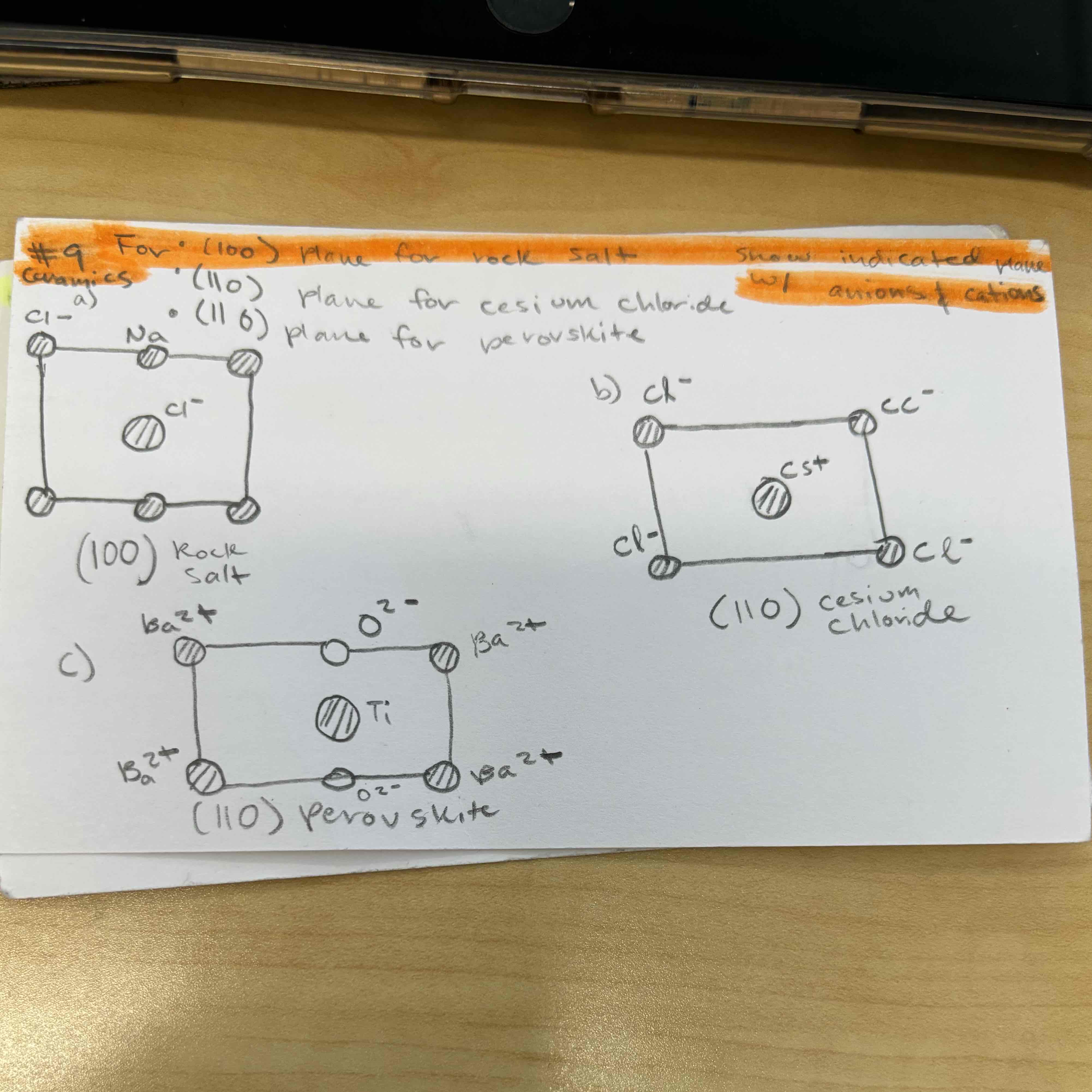
show indicated plane w anions and cations
4 characteristics of a polymer that impact both melting point and glass transition
chain double bonds
(ii) bulky side groups
(iii) polar groups.
(iv) molecular weights
cite 1 reason why ceramics are generally harder yet more brittle than metals
ceramics are made up of ionic and covalent bonds which are highly directional & do not easily slip. these bond types have limited slip systems and reduce dislocation making ceramica harder to deform and cause brittleness.
metals on the other hand, are made up of metallic bonds and which have significantly more slip systems and are therefore easier to deform
distinction between glass transition temp and melting temp
glass transition temp is where upon cooling, a noncrystalline ceramic or polymer transforms from a supercooled liquid to rigid glass
melting point on the other hand is where upon heating a material phase transforms into a liquid
SAMPLE PROBLEM
describe a typical polymer
a polymer is a chain like structure made up of repeating unit monomers. these monomers form a repeating long macromolecular chain, during polymerization reactive monomer sites, extend the chain
Name and briefly describe the 4 types of polymer molecular structures (pg 562)
linear: repeat units are joined together end to end in single chains
branched: structure w/ side-branched chains extending from primary chains
cross-linked: adjacent linear chains joined together by covalent bonds
network: multifunctional monomers w/ 3+ active covalent bonds; creates 3D networks
cite the differences in behavior between thermoplastic and thermosetting polymers
thermoplastics soften when heated and harden when cooled while thermosetting polymers are permanently hard and do not soften with heating
SCHEMATIC PLOTS OF THE 3 CHARACTERISTICS STRESS STRAIN BEHAVIORS FOR POLYMERIC
describe the mechanism for which elastomeric polymers deform elastically
Large elastic extensions are possible for elastomeric materials that are amorphous and lightly
crosslinked. Deformation corresponds to the unkinking and uncoiling of chains in response to an
applied tensile stress. Crosslinking is often achieved during a vulcanization process; increased
crosslinking enhances the modulus of elasticity and the tensile strength of the elastomer. Many
elastomers are copolymers, whereas silicone elastomers are really inorganic materials
discuss how the factors listed as bullet points, affect the polymer tensile modulus and/strength
molecular weight = increases as tensile strength increases
degree crystallinity = tensile modulus increases significantly as crystallinity increases
pre deformation= Stiffness and strength are enhanced by permanently deforming the polymer in tension
heat treatment = tensile modulus increases as annealing temp increases
what is Ohm’s Law and how is electrical resistivity related to electrical resistance
the ease with which a material transmits an electric current
electrical resistivity = (measure of material’s resistance to the passage of electric current) is related to resistance (how much the material used to make wire/component resists current) bc resistivity depends on resistance (the amount of current passing through a wire or lack thereof is inherently related to what the wire was made with)
list 4 characteristics that affect a polymers melting and glass transition temp
molecular weight
chemical bonds
degree crystallinity
chain stiffness
cite polymer application types and general characteristics of each
SEE TXTBOOK
How are materials classified as electrical conductors, semiconductors, or insulators
Metals are good conductors, typically having conductivities on the order of 107 (Ω·m)−1. electrical insulators are materials with very low conductivities, ranging between 10−10 and 10−20 (Ω·m)−1;. Materials with intermediate conductivities, generally from 10−6 to
104 (Ω·m)−1, are termed semiconductors.
In the electron energy band structure, what is an "energy gap"
In the band structure of electrons, one band is completely filled with electrons. If an empty conduction band exits separated from the valence band, an energy gap lies
between them
Schematically illustrate the temperature-dependence of electrical conductivity in a pure metal and
a pure semiconductor.
Distinguish between intrinsic and extrinsic semiconducting materials.
For intrinsic behavior, the electrical properties are inherent in the pure material, and electron
and hole concentrations are equal.extrinsic = Electrical behavior is dictated by impurities for extrinsic semiconductors. Extrinsic
semiconductors may be either n- or p-type depending on whether electrons or holes,
respectively, are the predominant charge carriers
For a p–n junction, explain the rectification process in terms of electron and hole motions.
…
Briefly describe the phenomena of ferroelectricity and piezoelectricity.
Ferroelectric materials exhibit spontaneous polarization
electric field is generated when mechanical stresses are applied to a piezoelectric material.
optical #2 briefly explain why metals are opaque to electromagnetic radiation having photon energies within the visible region of the spectrum.
optical #4 : Briefly explain what determines the characteristic color of (a) a metal and (b) a transparent nonmetal
Briefly explain why metallic materials are opaque to visible light.