8.2/8.3: Trends for Atomic Size, Ionization, and Electron Affinity
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
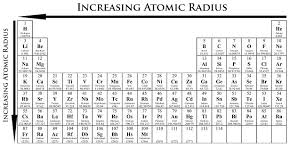
atomic size increases as n increases (True or false)
true
atomic size decreases/increases as Zeff increases
decreases
for main group elements, atomic size decreases/increases down a group in the periodic table and decreases/increases across a period
increases; decreases
what is ionization energy (IE)
how much energy is needed to remove an electron form an atom
atoms with low IE tend to form cations/anions
cations
atoms with high IE tend to form cations/anions
anions
Draw and tell trend of IE
decreases down a group, increases across a period
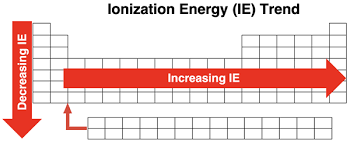
does it take more energy to remove core electrons than valence electrons
yes
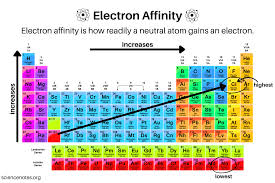
what is electron affinity (EA)
how likely an atom would accept an electrons
what does the # of low EA look like
it is closer to 0 or positive
low EA means electrons is less/more likely to accept an atom
less
high EA means electrons is less/more likely to accept an atom
more
what does the # of high EA look like
very negative number
Atoms with low EA tend to form cations/anions
cations
Atoms with high EA tend to form cations/anions
anions
draw and tell trend for atomic size
increases from right to left, increases down a group
draw and tell trend for electron affinity
not really a trend, has many exceptions. increases up a group, increase from left to right