Cells & Batteries
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
what are chemical/simple cells?
Chemical cells or simple cells are a source of electrical energy
describe the simplest design of chemical cells
The simplest design consists of two electrodes made from metals of different reactivity immersed in an electrolyte and connected to an external voltmeter by wire, creating a complete circuit
what is a common example of chemical cells?
zinc and copper
how is a chemical cell formed from zinc and copper? (4)
Zinc is the more reactive metal and forms ions more easily, readily releasing electrons
The electrons give the more reactive electrode a negative charge and sets up a charge difference between the electrodes
The electrons then flow around the circuit to the copper electrode which is now the more positive electrode
The difference in the ability of the electrodes to release electrons causes a voltage to be produced
why is zinc the more negatively charged electrode?
: When zinc loses electrons, it forms Zn²⁺ ions, which go into the solution:
These Zn²⁺ ions leave the zinc electrode, meaning the solid metal itself is left behind with extra electrons.
These extra electrons build up on the zinc electrode, making it negatively charged (even though the zinc atoms in solution are positive).
why is copper the more positively charged electrode?
The zinc electrode has extra electrons, making it negative.
The copper electrode gains electrons, but Cu²⁺ in solution takes them to form solid copper, so it doesn’t accumulate excess negative charge.
Since zinc has more free electrons than copper, copper becomes relatively more positive.
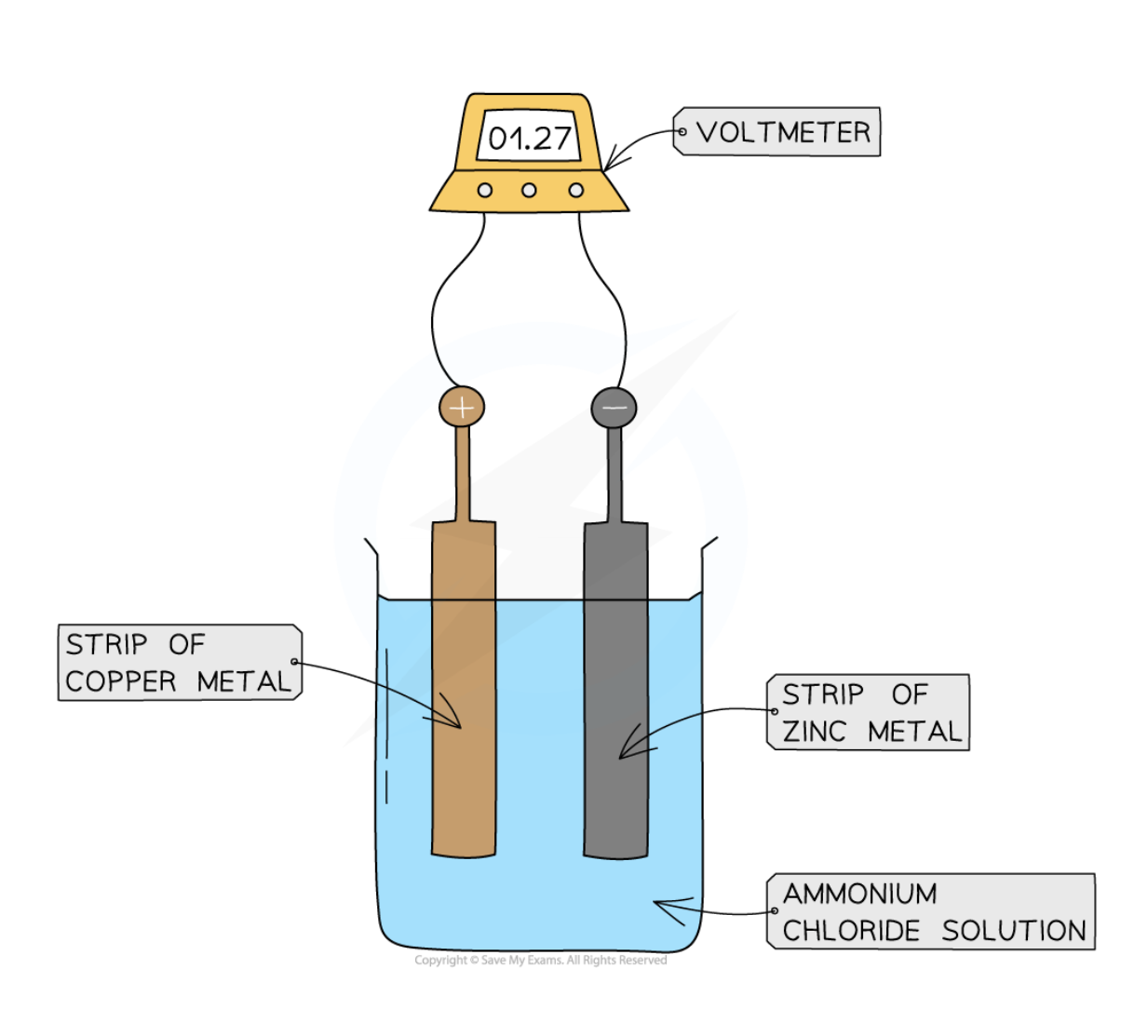
what 4 factors affect the amount of voltage produced by chemical cells?
1. Type of electrodes (metals with a larger difference in reactivity will produce a larger potential different)
2. Type of electrolyte (must be an aqueous ionic compound to conduct electricity)
3. Temperature of electrolyte (higher temperature, larger voltage)
4. Concentration of electrolyte (higher concentration, larger voltage)
how are electrochemical cells familiar?
Electrochemical cells include the familiar batteries used in everyday appliances and cars
how do batteries work?
Batteries work by connecting two or more cells in series, which combine to give a larger overall voltage
what happens to batteries over time?
Over time the electrodes degrade as the reactions that occur there are irreversible
for how long do cells produce voltage? what happens after that?
Cells produce a voltage only until one of the reactants is used up and when this occurs the battery dies or goes flat
The products formed cannot be reverted back into reactants as the reaction is irreversible and the battery must be replaced
in what type of batteries to they go flat?
in non-rechargeable batteries such as alkaline batteries
what happens in rechargeable batteries?
In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply
This reverses the chemical reactions taking place allowing the cycle to be
what do chemical reactions generate?
electricity
what do electrochemical cells use to create voltage?
electrochemical cells use chemical reactions to generate voltage
what happens when electrodes are connected by wire?
When the electrodes are connected by a wire, charge is able to flow between them. This generates an electrical current.
how can voltage be measured?
The voltage produced by a cell can be measured by connecting a voltmeter to the wire.
what are batteries formed for
batteries are formed by connected cells in series by wire to produce a high enough voltage to be useful
example of a rechargeable battery
lithium ion battery (for portable electronics)