Chem equations
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
absolute uncertainty
+-(1/2 x resolution of measuring equipment)
percentage uncertainty
absolute uncertainty/measurement x 100
Energy (Q) /J
m(g) x c (Jkg-1C-1) x T(.C)
No. of moles (n) /mol Molar mass
m/M
no. of moles (n) /mol Concentration
c(moldm-3) x v(dm3)
Relative abundance (AR)
(AR of isotope 1 x Abundance of isotope 1 + AR of isotope 2 x abundance of isotope 2)/100
Pressure(Pa) x Volume(m³)
n(mol) x R(8.31JK-1mol-1) x T(K)
Combustion equation
CXHy + (x+y/4)O2 ——> xCO2 + y/2H2O
Enthalpy change (^H)
-Q(J) / n(mol)
^H reaction
-Σ^Hf(reactants) - Σ^Hf(products) OR Σ^Hc(reactants) - Σ^Hc(products)
Equilibrium constant (Kc)
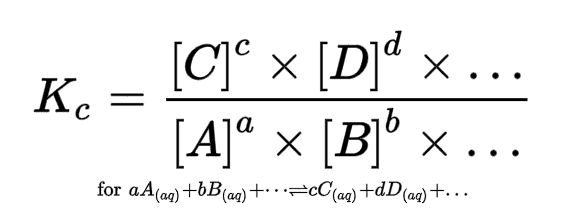
mole fraction (xA)
number of moles of A /total number of moles of gas
Partial pressure (total)
PA + PB.. etc
Partial pressure of A (pA)
mole fraction A(xA) x total pressure (PT)
Acid + metal →
salt and hydrogen
Acid + base →
salt + water
Acid + Carbonate →
salt + water + carbon dioxide