Module 6 Overall Flashcards
1/143
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
Immune system definition
collection of cells, tissues, and molecules that mediate resistance to infections and eliminate tumors
Immunology definition
study of the immune system and its response to invading pathogens and tumors
Immune response definition
Coordinated and tightly controlled reaction of the immune system to infectious microbes
Immunity definition
Resistance to disease, specifically those caused by bacteria, viral, fungal, or parasitic infections.
extended recently to include tumor immunity
Immunity not just about killing pathogen but also keep body in working order
What is the function of the immune system
prevent infections
eradicate established infections
detect and eliminate tumors as well as tolerate own cells
Congenital (primary) Immune defeiciency
genetic condition that results in a lack of key cells and molecules of the immune system
MORE RARE
Acquired (secondary) Immune deficiencies
infections that kill key immune cells and molecules
MORE COMMON
Overreacting immune system
a condition where the immune system responds excessively to harmless substances, leading to allergies, asthma, or autoimmune diseases
Immune system integrations with other systems
GI tract
Cardiovascular
Respiratory
Nervous
Endocrine
Skin
Mouth
Where do Immune cells originate and how do they travel
Originate in bone marrow
Travel via blood/lymph vessels
may travel/migrate or be resident in tissue
Autocrine
mechanism of action on self
Paracrine
mechanism of action on neighboring cells
Endocrine
mechanism of action over long distances
usually involves travelling via blood/lymph
Physical and Chemical barriers
Epithelial cells (skin, gut, resp. tract)
secretions (sweat, wax, tears)
mucous (nose, trachea)
urine
enzymes
stomach pH
Central immune sites
the lymphoid tissue/organ itself
Primary lymphoid organs
bone marrow
thymus
Secondary lymphoid organs
spleen
lymph nodes
mucosal associated lymphoid tissue
cutaneous associated lymphoid tissue
Peripheral immune sites
all other tissues and systems
skin, liver, gut, heart, brain, CNS, muscle, lungs, etc.
Thymus
Identifies as a primary lymphoid organ by Jacques Miller
Located right above heart and between lungs
Site of T cell maturation enable T cells to tolerate host cells
Differentiation of Immune cells
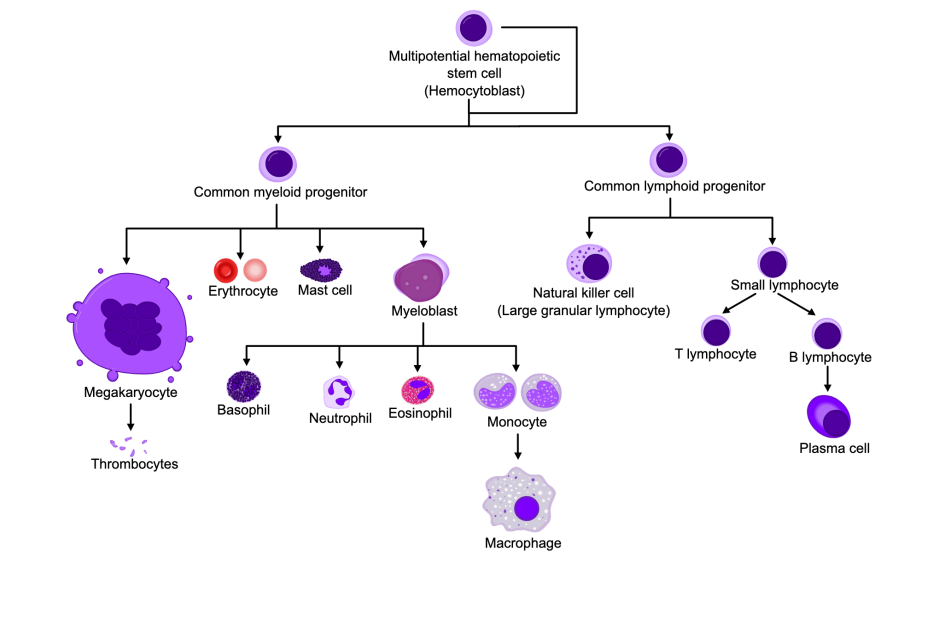
Common myeloid progenitor cells (and what they differentiate into)
Involved in the innate immune system
Differentiate into:
Megakaryocyte (form thrombocytes)
Erythrocytes
Mast Cells (inflammation)
Myeloblasts - produce:
Basophils
Neutrophils
Eosinophils
Monocytes
Basophils
release histamine and other chemical involved in inflammation and allergic responses
Neutrophils
most abundant type and are the first to respond to bacterial infections (via phagocytosis)
Eosinophils
allergic reactions and fighting parasites
Monocytes
become macrophages
Common lymphoid progenitor cells (and what they differentiate into)
involved in the adaptive immunity
Differentiate into:
Natural killer cells (large granular lymphocytes)
Small lymphocytes (T Cells, B Cells → plasma cells)
What lymphocyte?
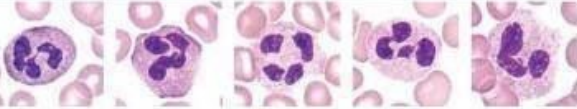
Neutrophils - have sgemented nucleus and granules in cytoplasm
nucleus divided into 2-5 segments giving multi-lobed appearance
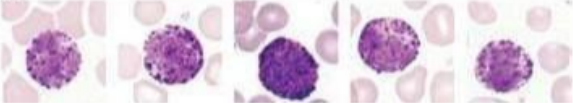
What lymphocyte?
Basophils - large, irregular shaped nucleus, lots of granules in cytoplasm that contain histamine
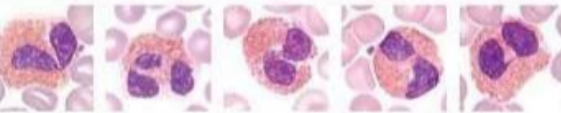
What lymphocyte?
Eosinophils - have bi-nucleus and large granules in their cytoplasm
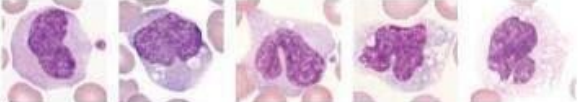
What lymphocyte?
Monocytes - large kidney shaped cells with single large oval shaped nucleus. Small amount of cytoplasm and can differentiate into macrophages
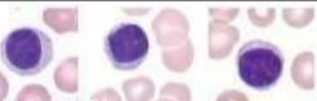
What lymphocytes?
T cells - have a round nucleus with a small amount of cytoplasm
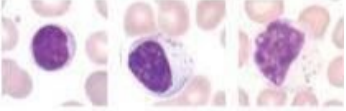
What lymphocytes?
B cells - have round nucleus with more cytoplasm than T cells
3 main types of T cells
Helper T cells
contain CD4+ receptors
activate other cells of the immune system
Cytotoxic T cells
contain CD8+ receptors
kill infected/cancerous cells
Regulatory T cells
prevent autoimmune reactions by suppressing the immune response
B cell action
Have B cell receptors (BCR) which recognize and bind to specific antigens - when BCR binds to antigen, B cells differentiate into plasma cells and produce antibodies to neutralize antigen
Innate Immunity
acts as first line of defence against invading pathogens
present at birth and provides immediate protection
Components of innate immunity
Epithelial barriers
cells in circulation and tissue
Phagocytes: neutrophils & acrophages
Exocytes: eosinophils, mast cells, basophils
Molecules
cytokines
blood preoteins
Mast cells
tissue resident cells - first responders to injury
reside in peripheral tissue and often exposed to environment (gut, lung, skin, oral mucosa)
How do mast cells respond to infection
degranulate contents (histamine, other soluble factors)
increase vascular permeability & promote inflammation
Cascade of events when danger is detected
Mast cells and macrophages release histamine, cytokines, protaglandins
dilated blood vessels allow for more blood flow and fluid to the area (redness + swelling)
carries innate immune cells and plasma proteins to the area
Induces expression of adhesion molecules on endothelial cells lining the blood vessels
attracts neutrophils to the area for:
phagocytosis
secretion of inflammatory cytokines
extend web-like extracellular trap for bacteria
Characteristics of innate immunity
Speed: early and rapid
Duration: short-lived (5 days in circulation, 2-6 hours in tissue)
Repetitive: responds the same way each time
Interactive: interacts with other cells of the innate and adaptive immune system
Non-reactive: is tolerant of host cells
How does the innate immune system distinguish between self and non-self cells
Uses Pattern Recognition Receptors (PRRs) expressed on cell surface to identify host cells
Detect Pathogen-associated Molecular Patterns (PAMPs) which are present on harmful invaders
Adaptive immune system
adapts to the presence of microbial invaders
consists of T&B cells that recognize specific antigens
uses effector and memory cells to coordinate immune response
Effector cells
detect and respond to antigens
Memory cells
cells that have encountered the pathogen before and can respond more effectively and rapidly the next time that the pathogen is encountered
B cells reason for name
Bursar of Fabricius
T cell resaon for name
mature in the thymus
Two main types of adaptive immunity
Humoral immunity
Cell-mediated immunity
Humoral immunity
Involves B cells that secrete antibodies (plasma cells) in response to extracellular pathogens
Cell-mediated immunity
Involves T cells (helper and cytotoxic) that help phagocyte microbes and kill infected cells
3 Main types of T cells
Helper T cells (Th)
Regulatory T cells
Cytotoxic T Lymphocytes
Helper T cells (Th)
help other cells perform their function
Ex, Help B cells produce antibodies, help cyto T cells kill
Mechanism:
CD4 → MHC2
Regulatory T cells
suppress or regulate the immune response to ensure self-tolerance and prevent autoimmunity
Cytotoxic T lymphocytes (CTLs)
kill target cells in highly specific way - they get help from helper T cells and play role in viral infections and anti-tumor immunity
Mechanism:
CD8 → MHC1
Immune cells in the gingiva
Neutrophils (continually transmigrate through epithelium)
T cells (resident lymphocytes)
B cells
Innate lymphoid cells
Mononuclear phagocytes
Summary of humoral and cell-mediated immunity
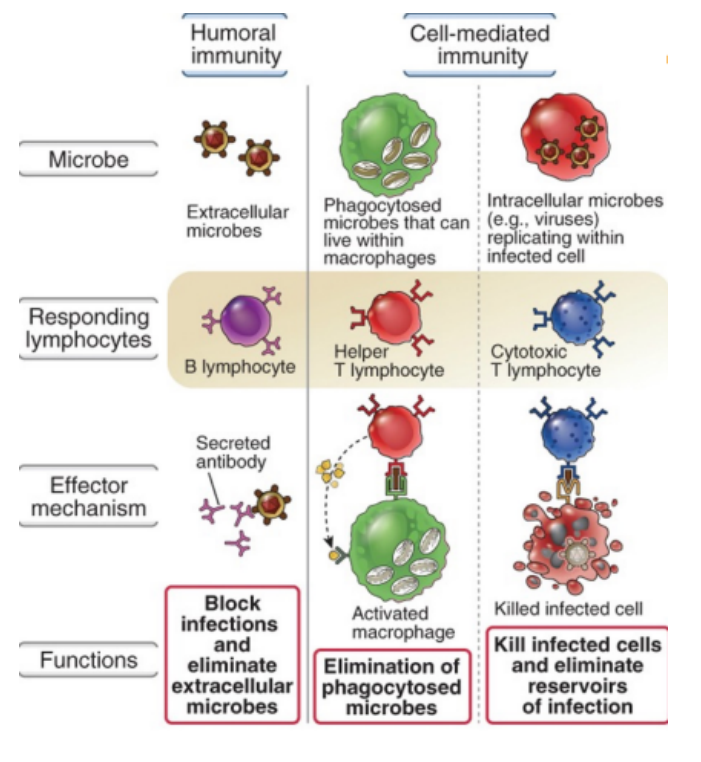
Chemokines
chemical messengers that are important for cell migration - direct cells where to go
Chemotaxis
The migration of leukocytes to a specific area
Formation of chemokine gradient that guides immune cells to sites of inflammation or injury
Process of leukocyte migration
Tissue resident cells promote inflammation
TNF and IL-1 stimulate endothelial cells the produce adhesion molecule (E-selectin and P-selectin)
Circulating phagocytes express surface carbohydrates that bind weakly to the E-selectin and P-selectin
causes cells to “roll” along endothelium
leukocytes express integrins on cell surface
resident cells produce chemokines
stimulates leukocyte integrins to bind ligands on endothelium
binding of integrin to ligands stops leukocytes from rolling
once leukocyte stops, it squeeze through the endothelium and into the extracellular space (diapedesis)
Primary features and functions of neutrophils
not normally found in tissues
recruited to inflamed peripheral sites
potent anitbacterial function
entraps bacteria in “Neutrophil Extracellular Traps” (NETs)
perform phagocytosis
secret cytokines to recruit other immune cells
promotes phagocytosisby macrophages
short-lived
Primary features and functions of macrophages
monocytes migrate into inflamed tissue and differentiate into macrophages
can be resident in tissue
Ex, Kupffer cells in the lover or alveolar macrophages in lung
secrete cytokines
often long-lived
act as antigen presenting cells
Phagocytosis
often triggered by microbes binding to phagocyte receptors → once microbe binds to cell phagocyte will form plasma membrane around microbe (phagosome) → phagosome fuses with lysosomes = phagolysosomes → lysosomes kill microbes
PRRs binding to PAMPs
Activates:
transcription factors that stimulate expression of gene encoding cytokines, enzymes, proteins involved in antimicrobial function
Toll-like receptors are activated
phagocytosis and production of soluble mediators
Toll-like receptors (TLR)
type of PRR on host cells
discovered by Bruce Beutler and Jules Hoffman in Drosophila flies
Locations of TLRs
on Cell surface: recognize extracellular microbes
on Endosomes in cell: recognize ingested microbes
Common types of TLRs
TLR 5: flagella
TLR 2 and 6: recognize diacylinpopeptide
TLR 1 and 2: recognize triaclipopeptide
TLR 4: LPS of gram neg bacteria
TLR 3,7,8,9: located on endocytic vesicles
Nucleotide oilgomerization domain family (NOD proteins)
family of PRRs that can detect pathogens in cytosol and signal pathogen presence to immune system
Abundance of leukocytes
Neutrophils > Lymphocytes > Macrophage/monocytes > Eosinophils > Basophils (most to least abundant)
Never Let Monkeys Eat Bananas
Primary features and functions of Eosinophils
Contains enzymes harmful to cell walls of parasites and helminths (contents can also harm host cells)
some found in peripheral tissue (resp, GI, genitourinary)
increase immune recruitment
participate as effector cells in adaptive immune response
Primary features and functions of basophils
not normally found in peripheral tissue
recruited from the bloodstream during inflammation
supporting role in the development of the adaptive immune system
Innate lymphoid cells (ILCs)
“lymphocyte-like” cells that live in epithelial tissue and produce cytokines
acts similar to T cells
important function in oral mucosa
Main function of ILCs
provide early defence against infections
interact with the cells of the adaptive immune system to guide T cells
Major groups of ILCs
Group 1 ILCs (ILC1): defence against pathogens
Group 2 ILCs (ILC2): promote allergic inflammation in skin and airways
Group 3 ILC (ILC3): mucosal barrier function
Natural Killer (NK) cells
subset of ILC 1
class of innate lymphocytes
secrete cytokines to kill infected and stressed cells
Complement system
collection of circulating and cell membrane proteins that are important in host cell defence and antibody-mediated tissue injury
assists the antimicrobial activity of the immune system
Activation of Complement System
sequence of cleavage of complement proteins → triggers effector molecules to kill microbes (can be innate or adaptive)
3 pathways of complement activation
Alternative
Lectin
Classical
All three pathways produce C3b, C3a, C5b, C5a, MAC
C3b
coats bacteria to be recognized by type 1 complement receptor (CR1) so it can be phagocytosed or killed
C3a and C5a
anaphylatoxins that stimulate inflammation and recruits neutrophils to tissue sites
Alternative and lectin pathways
initiated in the absence of antibodies = Innate immune system
Classical pathway
initiated when antibodies attach to antigens = adaptive immune system
Antibiotic definition
a chemical substance produced by microorganisms that have the capacity to inhibit growth and destroy bacteria
Antimicrobial Resistance (AMR)
changes in bacteria and viruses that make the no longer respond to medicine → difficult to treat
How are antibiotics classified
What part of the cell they target
Whether the drug kills of inhibits the cell (bacteriolytic/bacteriostatic)
Range of action
Narrow
Moderate
Broad
Species the drug works on
Indications for antibiotic use in dentistry
Prophylaxis - preventing bacterial infections before dental procedures in at-risk patients
Treatment - usually always required alongside dental surgery
Post-operative - to reduce risk in medically compromised patients
Antibody prophylaxis in dentistry
1hr before treatment for people with cardiac conditions (congenital heart disease, previous infective endocarditis, prosthetic cardiac valve)
Antibiotics for treatment in dentistry
Used for:
odontogenic infections
necrotising periodontal disease
peri-implantitis
salivary gland infections
Antibiotics for postoperative in dentistry
Use if patient is:
immunocomprimised
past history of infection
experiences systemic disorders (fever, tachycardia, sweating, etc)
Recommended antibiotics for odontogenic infections
Metronidazole + amoxicillin
Amoxicillin + clavulanate
Recommended antibiotics for necrotising periodontal disease
Metronidalzole
Recommended antibiotics for prophylaxis
Amoxicillin or Cefelexin or Clindamycin (depending on patient allergy)
Principles of prescribing antibiotics
use appropriate antibiotic for presenting infection
Follow Antibiotic Creed (MIND ME)
M: microbiology guides therapy wherever possible
I: indications should be evidence-based
N: narrowest spectrum required
D: dosage appropriate to the site and type of infection
M: minimize duration of drug
E: ensure monotherapy in most situations
Common antibiotics
Phenoxymethylpenicillin (PenV) - used for majority of odontogenic infections
Amoxicillin - most prescribed antibiotic
Metronidazole - second most prescribed
Amoxicillin + Clavulanic acid - third most prescribed
Innate vs. Adaptive Immunity
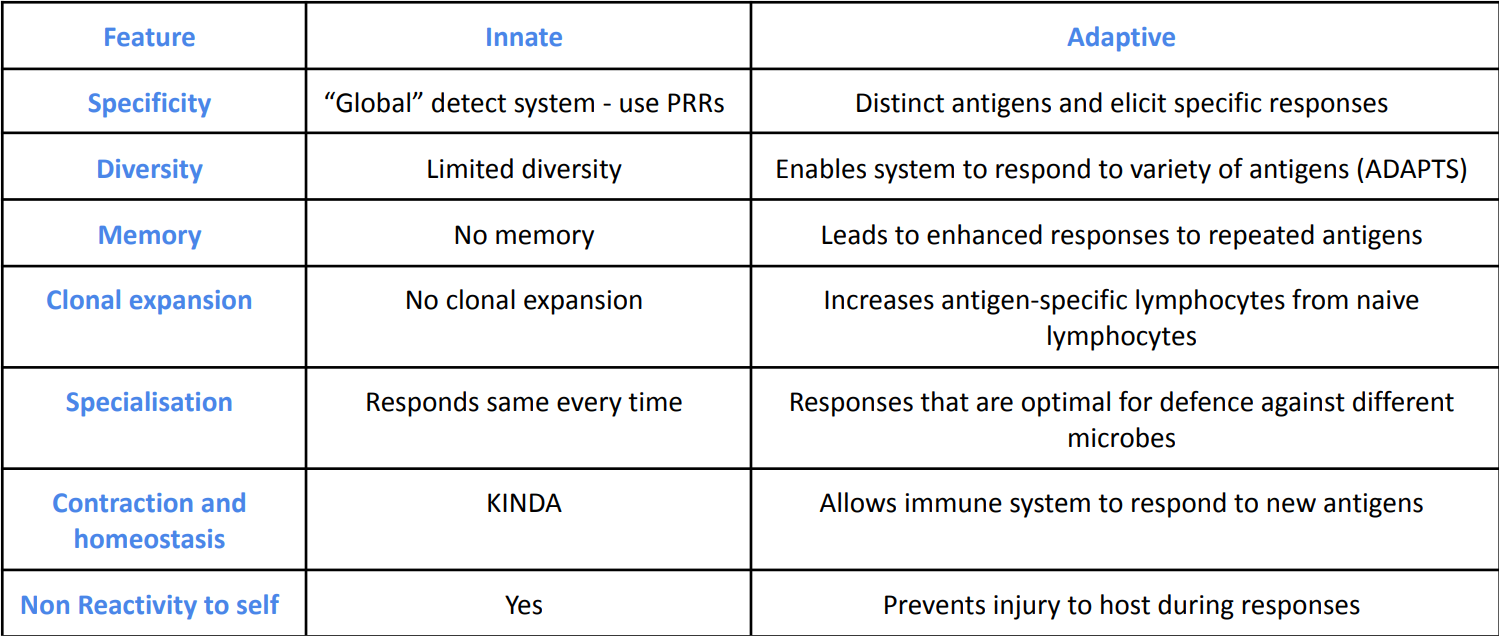
Thymic output
the production and release of T cells from the thymus gland into the bloodstream
output is highest during early life - peaks around 20-30 yrs old
Graph of Thymic output, Memory T cells, Native T cells
Clonal Expansion
Feature of adaptive immunity that involves rapid proliferation
Specific clonal expansion occurs for a specific antigen
Process of clonal expansion
T cells activated in response to infection
T cells trigger clonal expansion (24-48 hours)
produce tons of CD8+ and CD4+ to treat infection
following treatment of infection remaining T cells become memory cells
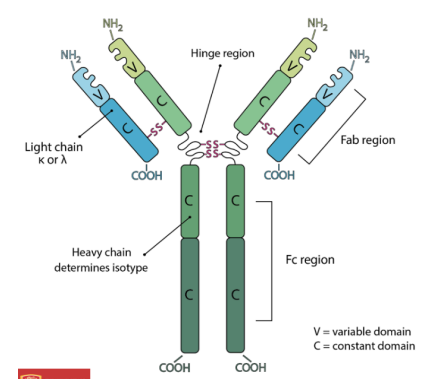
Basic antibody structure
Variable region = different for each antibody - gives antibodies their specificity
Constant Region = same for each class of antibody - determines the isotype of antibody (IgM, IgA, etc)
Hinge region: offers light and heavy chain flexibility
Classes of anitbodies
MAEDG (pentamer, dimer, rest are monomers)
IgM
IgA - neutralizes antigen
IgD - capacity to differentiate into any antibody - anchored to cell membrane, therefore not found in serum
IgE
IgG - neutralizes antigen - capacity to differentiate into any antibody - most abundant in blood
Mechanism of antibody action
blocking penetration of microbe through epithelial barrier
blocks binding of microbe and infection of cells
blocks binding of toxin to host cell receptor