5 - intracellular compartments and protein transport
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
true/false: nuclear membranes and the ER may have evolved through invagination of the plasma membrane
true
true/false: mitochondria are thought to have originated when an aerobic bacterium was engulfed by a larger anaerobic cell
true
explains why they have two membranes, have their own genomes, and don’t participate in the vesicular traffic that connects the compartments of the endomembrane system
mechanisms by which membrane enclosed organelles import proteins
transport through nuclear pores - stays folded
transport across membranes - protein unfolds
transport by vesicles - stays folded
N-terminal signal sequence
proteins destined for the ER
no signal sequence
stay in cytosol
if a signal sequence is removed from an ER protein and is attached to a cytosolic one…
both proteins are reassigned to the expected and inappropriate location
nuclear pore complex
forms a gate through which selected macromolecules and larger complexes enter or exit the nucleus
protein fibrils
protrude from both sides of the pore complex and converge to form a basket like structure on the nuclear side with spaces wide enough to not obstruct anything
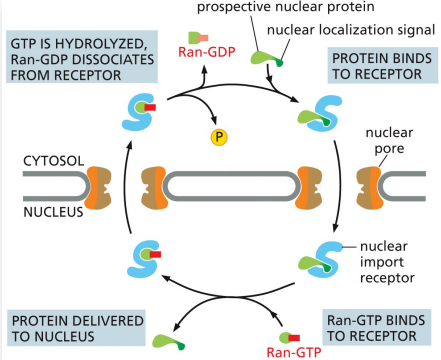
prospective nuclear proteins contain a nuclear localization signal…
that is recognized by nuclear import receptors to import them from the cytosol through the nuclear pores
what drives nuclear transport?
energy from gtp hydrolysis
Ran
monomeric GTPase with two conformations
one carrying GTP
one with GDP
Ran-GAP
in the cytosol, triggers GTP hydrolysis and converts Ran-GTP to Ran-GDP
Ran-GEF
in the nucleus, causes Ran-GDP to release its GDP and take up GTP
localization of Ran-GAP and Ran-GEF guarantees…
the concentration of Ran-GTP is higher in the nucleus, driving the nuclear import cycle
nuclear transport process
nuclear import receptor picks up a prospective protein in the cytosol and enters the nucleus
Ran-GTP binds to the receptor, causing it to release the nuclear protein
the receptor (still with Ran-GTP) is transported back through the pore to the cytosol where Ran hydrolyzes its bound GTP
Ran-GDP falls off the import receptor
Ran-GDP is carried into the nucleus via its own unique import receptor
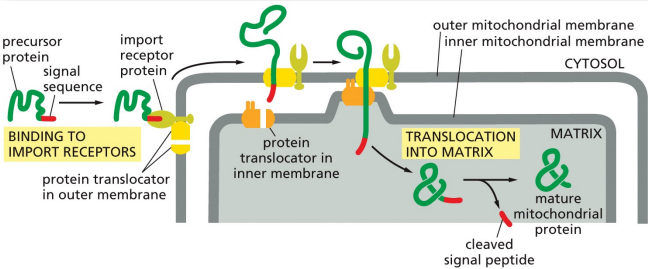
mitochondrial precursor proteins are unfolded during import bc…
a mitochondrian has an outer and inner membrane
import of mitochondrial precursor proteins
the mitochondrial signal sequence on a mitochondrial precursor protein is recognized by a receptor in the outer mitochondrial membrane
receptor is associated with a protein translocator which transports the signal sequence across the outer mito membrane to the intermembrane space
the complex of receptor, precursor protein, and translocator then diffuses laterally in the outer membrane until the signal sequence is recognized by a second translocator in the inner membrane
the two translocators transport the protein across both the outer and inner membranes, unfolding the protein in the process
the signal sequence is cleaved off by a signal peptidase in the mito matrix
ribosomes that are translating proteins with no ER signal sequence…
stay free in the cytosol
ribosomes that are translating proteins w/ an ER signal sequence
will be directed to the ER membrane
polyribosome
ribosomes bind to each mRNA molecule
what two factors direct a ribosome to the ER membrane?
an ER signal sequence and an SRP direct
SRP
binds to both the exposed ER signal sequence and the ribosome, slowing protein synthesis by the ribosome
SRP-ribosome complex
binds to an SRP receptor in the ER membrane. SRP is released and the ribosome passes from the SRP receptor to a protein translocator in the ER membrane. Protein synthesis resumes and the translocator starts to transfer the growing polypeptide across the lipid bilayer
a soluble protein crosses the ER membrane and enters the lumen
protein translocator binds the signal sequence and threads the rest of the polypeptide across the lipid bilayer as a loop
the signal peptide is cleaved from the growing protein by a signal peptidase. the cleaved sequence is ejected into the bilayer where it is degraded
once protein synthesis is complete, the translocated polypeptide is released as a soluble protein into the ER lumen, and the protein translocator closes
a single pass transmembrane protein is retained in the lipid bilayer
an N-terminal ER signal sequence initiates transfer
the protein also has a second hydrophobic sequence which acts as a stop-transfer sequence
when this sequence enters the protein translocator, the growing polypeptide chain is discharged into the lipid bilayer
the N-terminal signal sequence is cleaved off, leaving the transmembrane protein anchored in the membrane
protein synthesis on the cytosolic side then continues to completion
a double pass transmembrane protein has an internal ER signal sequence
this internal sequence acts as a start-transfer signal and anchors the final protein in the membrane
the internal signal sequence is recognized by an SRP which brings the ribosome to the ER membrane
when a stop transfer sequence enters the protein translocator, the translocator discharges both sequences into the lipid bilayer
neither the start transfer nor the stop transfer sequence is cleaved off, and the entire polypeptide chain remains anchored in the membrane as a double pass protein