4. PHAR4813 Regulations (73)
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
History of Pharma Industry
see image
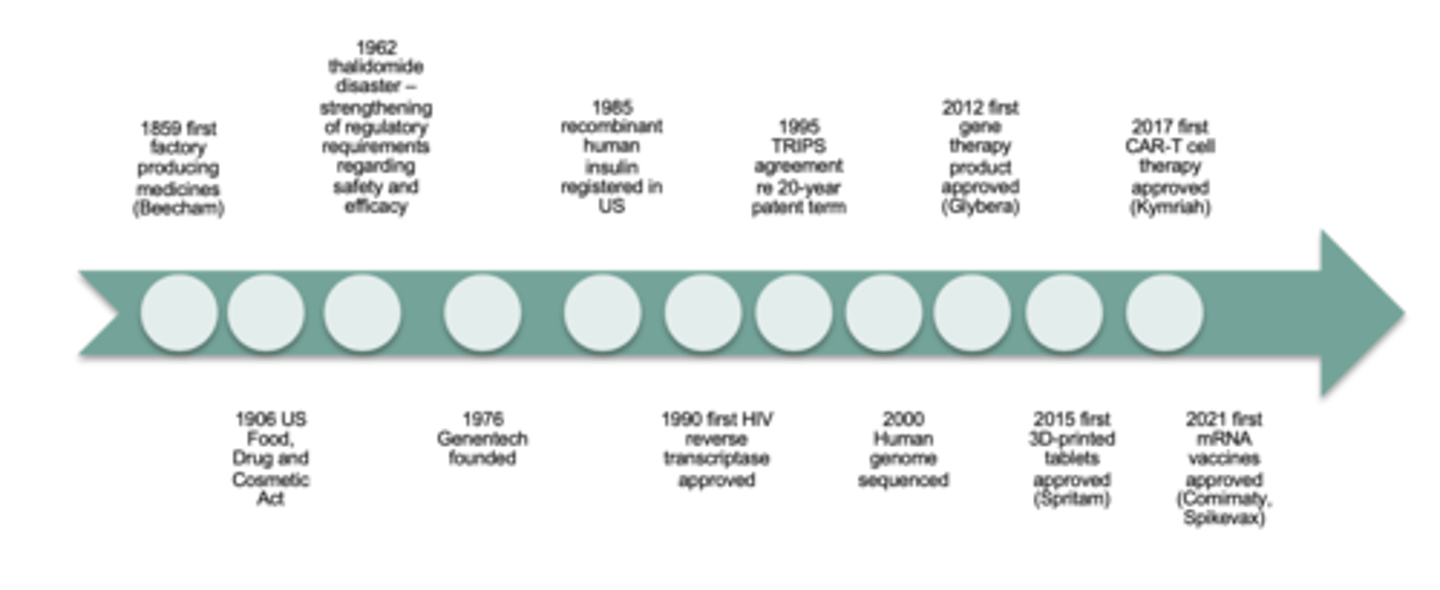
Current directions and influence on pharm industry - SCIENCE (6)
1. oncology
2. rare diseases
3. cell and gene therapy
4. mRNA-based therapies
5. Ai in drug development
6. precision medicine and data
Current directions and influence on pharm industry - POLICIES (7)
1. WHO: universal health coverage
2. WHO: future pandemic preparedness (pandemic treaty)
3. sovereign manufacturing capability
4. industrial and innovation
5. health funding and regulatory
6. science and research
7. export and trade
Current directions and influence on pharm industry - CORPORATE (8)
1. VALUE
2. ESG AND climate change impacts
3. EDI and increasing the patient voice
4. new ways of working
5. digital integration
6. codes of conduct and ethics
7. transparency
8. business and corporate policies
What are the top 3 therapeutic classes by sales (2023)?
1. anti-cancer, immunological
2. anticancer, other
3. gene therapy
What are the top 3 medtech products (2021)?
1. IVD (in vitro devices) i.e. Covid-19 antigen test kits, gene screening
2. cardiology
3. medical imaging
Explain the expenses and revenues curve for a new medicine
1. there is a huge cost in researching and designing a new drug initially
2. once product is registered and marketed, it reaches a break-even point and then has a huge spike in sales until it reaches a point of peak sales (during period of return)
3. once the 20-year patent expires, the influx of generic competitors causes a sharp decline in sales*
*company can choose to sell product to a generic company and reinvest into developing new therapeutic products

How much does it typically cost to bring a new product to market?
$1-3 billion
How long does it typically take to get a new pharmaceutical product to market?
12-16 years
List in chronological order the development pathway for new drugs (5)
1. drug discovery, development and pre-clinical studies (GLP, GMP, GCP)
2. clinical trials (GLP, GMP, GCP)
3. registration
4. reimbursement
5. marketing, pharmacovigilance, further Clinical Studies
GLP = Good Laboratory Practice; GMP = Good Manufacturing Practice; GCP = Good Clinical Practice; GVP = Good Vigilance Practice; PV = pharmacovigilance

Outline the Product Development Plan pyramid (9)
1. situational analysis
2. competitor landscape
3. target product profile
3. pre-clinical studies plan
4. clinical development plan
5. formulation and manufacturing
6. regulatory strategy
7. health technology assessment strategy
8. timeline
Outline the development pathway for medical devices (10)
1. initiation
2. formulation
3. design and development
4. verification and validation
5. manufacturing and teseting
6. clinical development
7. regulatory submissions
8. launch
9. post-market compliance
10. reimbursement
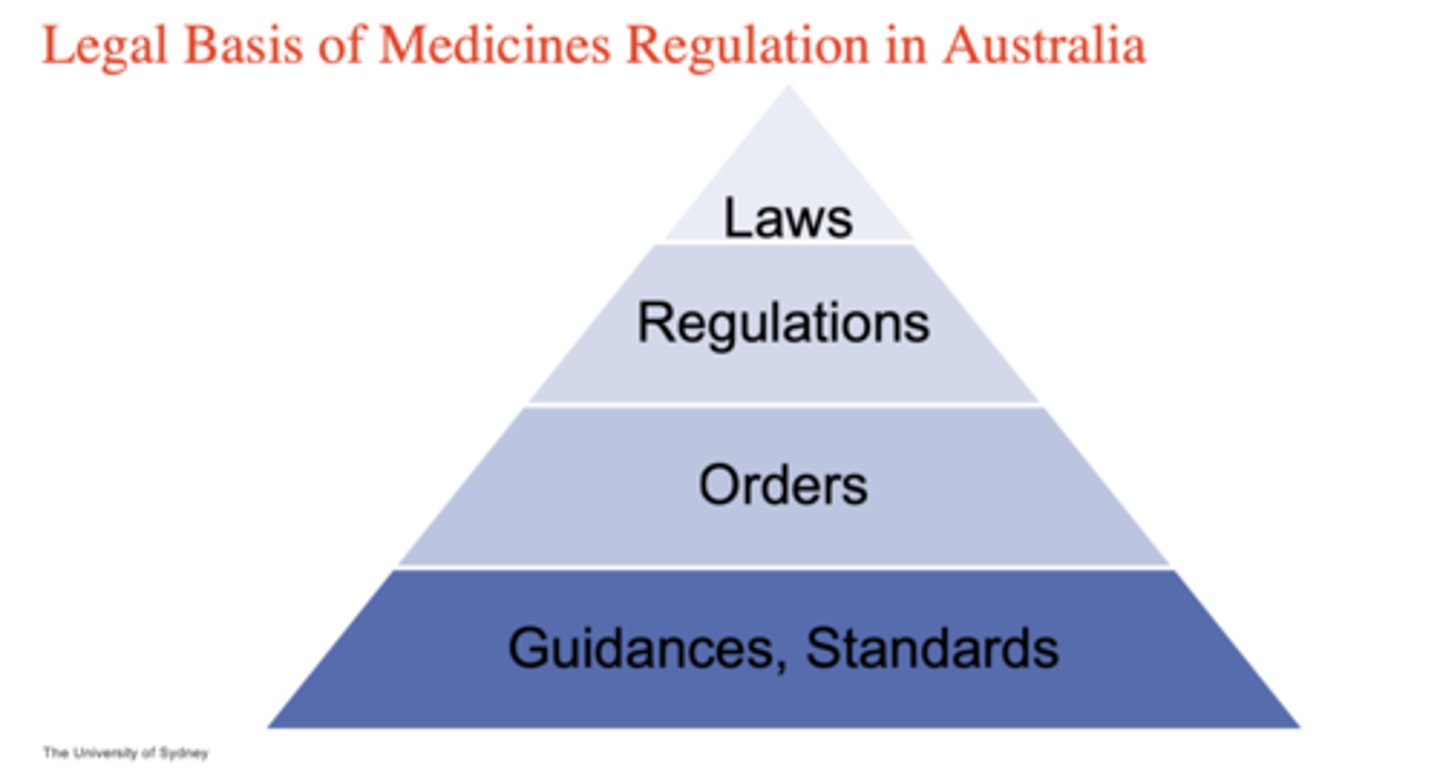
What is the legal basis of medicines regulation in Australia PYRAMID (4)
1. laws*
2. regulations
3. orders
4. guidances/standards
*bills are first drafted by government lawyers, drafts put before parliament to be reviewed and amended by lower house (house of representatives), then review and amend by Senate. Once both house approved and governor general signs it off
--> becomes act of parliament and legally binding*
Why does the development of medical devices take a shorter amount of time to get approved? (4)
- medical devices adhere to engineering manufacturing standards and engineering quality standards
- therefore, development is more rapid (3-7 years)
- lifespan of medical device is shorter on market as companies will continuously improve device with updates and refinements
- claim through MSAC
How many times has the Therapeutic Goods Act (1989) been amended?
85 times
What is the objective of the Therapeutic Goods act (1989)? (2)
1. to provide a national framework for the regulation of therapeutic goods so as to ensure their quality, safety, efficacy and timely availability
2. supported by a number of legislative instrument and guidelines
What does the Therapeutic Goods Act do? (4)
- establish the therapeutic goods administration (TGA) as the regulatory authority in Aus
- establish a number of advisory committees that assist the TGA in decision-making process
- regulates advertising of therapeutic goods
- licensing and inspection of manufacturers in Australia and overseas

What is the role of the TGA (5)
- pre-market evaluation and approval of therapeutic goods
- licensing of manufacturers (GMP)
- post-market surveillance through sampling, adverse effect reporting etc.
- development, maintenance and monitoring systems for listing of medicines
- assessment of medicines for export
What is the definition of a therapeutic good?
a good which is represented in any way to be, or is likely to be taken to be, for therapeutic use (unless specifically excluded or included under Section 7 of the Therapeutic Goods Act 1989)
What is the meaning of 'therapeutic use' (6)
- preventing, diagnosing, curing or allievating a disease, ailment, defect or injury
- influencing, inhibiting or modifying a physiological process
- testing the susceptibility of persons to a disease or ailment
- influencing, controlling or preventing conception
- testing for pregnancy or
- replacement or modification of parts of the anatomy
What are the classes of therapeutic goods? (7)
- prescription medicines
- over-the-counter or non=prescription medicines
- complementary medicines
- sunscreens
- medical devices and in vitro diagnostics (IVDs)
- biologicals
- blood and blood components
What is the significance of the ARTG?
- Australian Register of Therapeutic Goods (ARTG)
- all goods must be entered in the ARTG before they can be supplied in, imported to or exported from Australia
What are features of a registered medicine in Australia? (4)
- higher risk medicines that are registered on ARTG'
- evaluated for quality, safety and efficacy
- product information is approved by the TGA
- includes:
- ALL prescription medicines
- Most OTC medicines
- some complementary medicines
What are features of a listed medicine? (4)
- lower risk medicines that are listed on the ARTG
- contain pre-approved, low-risk ingredient
- can only make limited claims and cannot imply that they will be useful in the treatment of prevention of serious illnesses
- includes:
- some OTC medicines
- most complementary medicines
What are the features of medical devices as listed on the ARTG? (3)
- higher risk devices are evaluated for quality, safety and performance
- lower risk devices are not evaluated for performance
- devices are classified according to their level of risk, ranging from class 1 (lower risk) such as urine collection bottles to class 3 (higher risk) such as antibiotic bone cement
What is considered PRESCRIPTION medicine? (5)
- new chemical entities: small molecule products
- new biologic entities: MABs
- vaccines recombinant products
- plasma derived products
- gene therapy - in vivo delivery: encapsulated in a viral vector or lipid nanoparticle
What are the 4 main steps that must be submitted to the National Regulatory Authority of the relevant country?
- manufacturing process
- pre-clinical data
- clinical data
- product development
What is the process for a drug to get registered in Australia? (11)
1. applications by sponsor - legal Australian entity
2. review guidelines, orders, etc.
3. obtain Australian approved name (AAN)
4. obtain GMP certification
5. scheduling (PM are S4 or S8)
6. pre-submission meeting
7. obtain designations or determinations
8. write Au-specific Module 1
9. review dossier
10. publish dossier: eCTD format
11. submit dossier and fees
Why does a drug company need to have an ABN?
the drug company must have a base in Australia even if company originates from a different country because by law, Australia can only hold companies accountable if they have an Australian representative
What is the standard registration pathway for PRESCRIPTION medications? (6)
1. designation
2. submission
3. first round evaluation (TGA clock start)
4. second round evaluation (TGA clock stop for Sponsor responses)
5. expert advice
6. decision (TGA clock stop = 255 working days)

Comparing registration pathway for STANDARD/PROVISIONAL APPROVAL/PRIORITY REVIEW for new drugs
see table

Comparing designations and determinations for ORPHAN/PRIORITY REVIEW/PROVISIONAL APPROVAL
see table

What is the definition of a medical device?
any material, appartus, appliacnce, implant etc., including any component part or accessory including software, and in-vitro diagnostics, which is used in healthcare
How are medical devices classified?
risk-based i.e. the higher the risk, the higher the class
What are the classes for medical devices?
see table

What are the ESSENTIAL PRINCIPLES for medical device registration? (3)
- fundamental regulatory requirements
- listed in Schedule 1, Therapeutic Goods (Medical devices) Regulations 2002
- composed of:
- general principles to show benefits outweigh risks
- principles regarding design and construction
- principles regarding labelling
- principles regarding standards and testing of devices
What are the features of Conformity Assessment Procedures? (6)
- Schedule 3, Therapeutic Goods (Medical Devices) Regulations 2002
- Help demonstrate compliance with the Essential Principles
- Quality management System requirements e.g. ISO 13485
- Design examination
- Third party assessment
- Manufacturer Evidence:
- Declaration of conformity
- Conformity assessment certificate (TGA, EU notified bodies, Medical Device Single Audit Program, Japanese QMS certificate, US FDA PMA, HSA Singapore assessment)
What are the steps involved in SUPPLYING a medical device in Australia? (7)
- confirm your product is a medical device that needs to be included in the ARTG
- determine the kind of medical device - check GMDN code
- determine the classification of the medical device - which class?
- prepare all documents required for medical device inclusion:
- declaration of conformity
- manufacturer's evidence (compliance with Essential Principles)
- submit application for inclusion
- print out your ARTG certificate
- supply your device
What is the definition of an in vitro diagnostic (IVD) medical device? (3)
a) a reagent, calibrator, control material, kit, specimen receptacle, software, instrument, apparatus, equipment or system, whether used alone or in conjunction with another diagnostic product for in vitro use; and
b) intended by the manufacturer to be used in vitro for the examination of a specimen derived from the human body, solely or principally for:
i. giving information about a physiological or pathological state or a congenital abnormality; or
ii. determining safety and compatibility with a potential recipient; or
iii. monitoring therapeutic measures; and
c) not a product that is:
i. intended for general laboratory use; and
ii. not manufactured, sold or presented for use as an IVD medical device
IVD regulation. by TGA classification (4)
- Class 1 = no public health risk or low personal risk
- Class 2 = low public health risk or moderate personal risk
- Class 3 = moderate public health risk or high personal risk
- Class 4 = high public health risk
IVD regulation by TGA steps (40
- Step 1: identify the class of IVD
- Step 2: obtain GMDN and declaration of conformity from manufacturer
- Step 3: prepare and submit TGA conformity assessment
- Step 4: submit IVD application to TGA
What is GMDN?
an international agency used to name and group medical devices
CASE STUDY - timeline for clinical development of larotrectinib and entrectinib
Trk inhibitor development
- 1982- discovery of TPM3-NTRK1 gene as oncogene (cancer-causing)
- 1989- discovery of TrkA protein receptor as tyrosine kinase
- 1991 - characterisation of TrkA function as NGF receptor
- 2012 - Entrectinib enters clinical trials
- 2014 - Larotrectinib enters clinical trials
- 2018 - Larotrectinib FDA approved
- 2019 - Entrectinib FDA approved
- 2020 - FDA approval of companion diagnostic for larotrectinib*
in vitro diagnostic teset for expression of TPM3-NTRK1 gene on cancer cells of patient prior to starting treatment with larotrectinib
What are Trk inhibitors and how do they work? (4)
- they are small molecules that block receptors expressed on cancer cells
- preferentially block the aTP binding site of Trk A, B and C proteins
- inhibits catalytic activity and autophosphorylation, activation and downstream pathways
- pathway is involved in tumour genic progression of cancer cells --> blockage prevents cancer from spreading
Outline the clinical trial phases of Trk inhibitors (entrectinib and larotrectinib) PHASE 1 (3)
- first in human study to show that drug works for this gene mutation. Mutation in Trk protein turns on 100% of the time, not just after ligand binding
- first in human studies for oncology products are always with patients with a cancer, not healthy individuals
- also looking at tumour types
Outline the clinical trial phases of Trk inhibitors (entrectinib and larotrectinib) PHASE 2 (2)
- figuring exact dosage and formulation
- looking at different types of solid tumoups
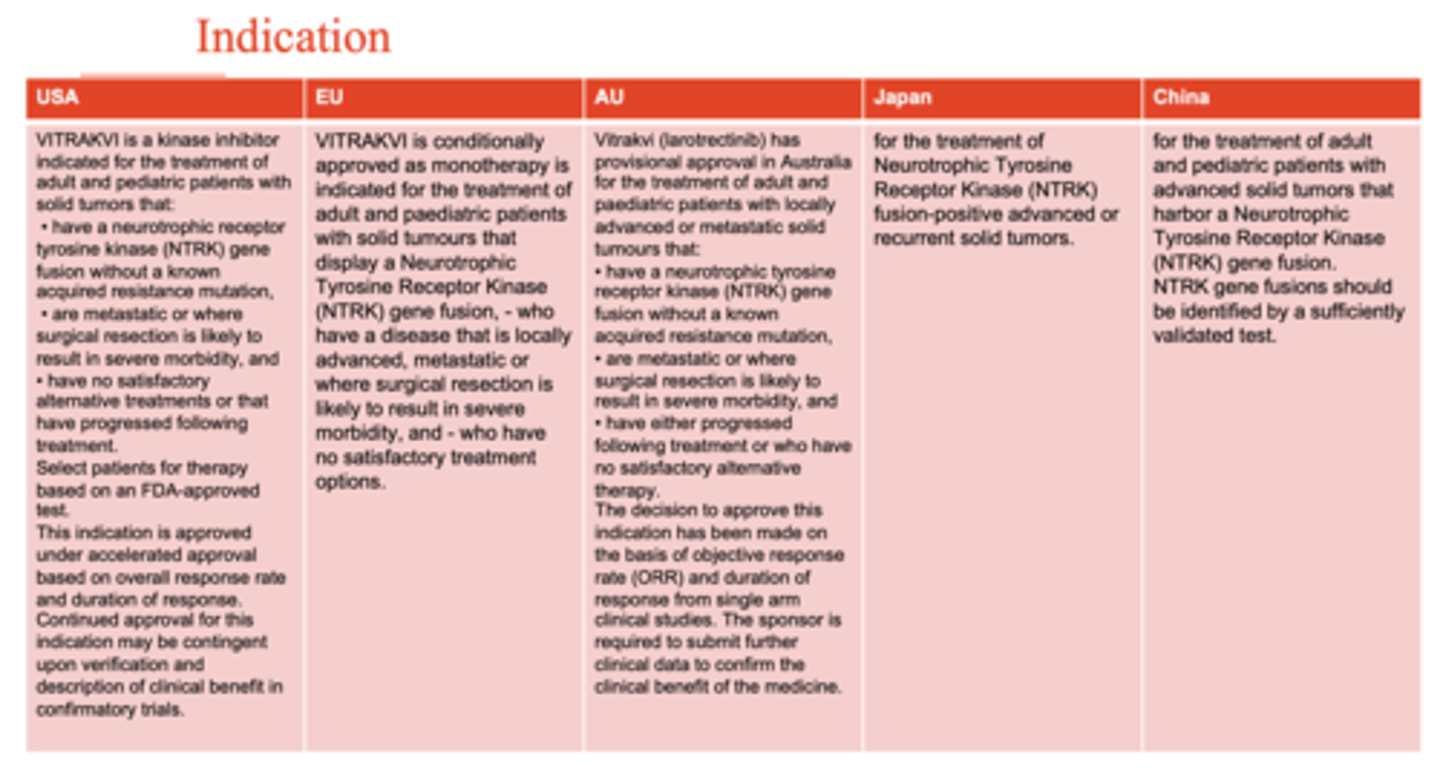
What are the indications that Trk inhibitors (entrectinib and larotrectinib) are approved for? (3)
- indicated for both adult and paediatric patients NTRK expressing gene solid tumour where it had metastasised or when surgical extraction is not beneficial to patient
- no satisfactory alternative treatment
- patient is selected after in vitro diagnostic test for NTRK gene expression
What is the approval status of Viktravi (larotrectinib) in Australia?
Larotrectinib has PROVISIONAL APPROVAL in Australia for the treatment of adult and paediatric patients with locally advanced or metastatic solid tumours that:
1. have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion without a known acquired resistance mutation
2. are metastatic or where surgical resection is likely to result in severe morbidity, and have EITHER:
- progressed following treatment or
- who have no satisfactory alternative therapy
3. the decision to approve this indication has been made on the basis of objective response rate (ORR) and duration of response from single arm clinical studies.
*the sponsor is REQUIRED to submit further clinical data to confirm the clinical benefit of this medicine*
Co-dependent health technology of entrectinib and larotrectinib features (3)
- companion diagnostic test needs to go through Medical Services Advisory Committee (MSAC) before they can be approved
- drug was reimbursed on PBS
- in vitro was reimbursed through MBS
What is the definition of a biological? (6)
a biological comprises, contains or is derived from HUMAN CELLS or HUMAN TISSUES (or specified by the Secretary to be a biological)
AND IS UESD TO:
1. treat or prevent disease, ailment, defect or injury; or
2. diagnose the condition of a person; or
3. influence, inhibit or modify a physiological process in persons; or
4. test the susceptibility of persons to a disease or ailment; or
5. replace or modify parts of the anatomy in persons
Biologicals are grouped into classes based on levels of what?
risk
What are Class 1 biologicals?
FMT products (in hospitals)
faecal matter transplant
What are Class 2 biologicals?
acellular skin for wound covering (tissue banks)
What are Class 3 biologicals? (2)
- mesenchymal stem cell for treatment of graft-versus-host disease
- demineralised bone mixed with carrier
What are Class 4 biologicals? (2)
- iPSC-derived cell therapies
- CAR-T cells
What is the application process for Class 1 biologicals? (5)
- must be listed in Schedule 16 of Therapeutic Goods Regulations, 1990
- currently only faecal microbiota transplant (FMT) material listed
- submit application form
- pay appropriate fees
- GMP NOT required
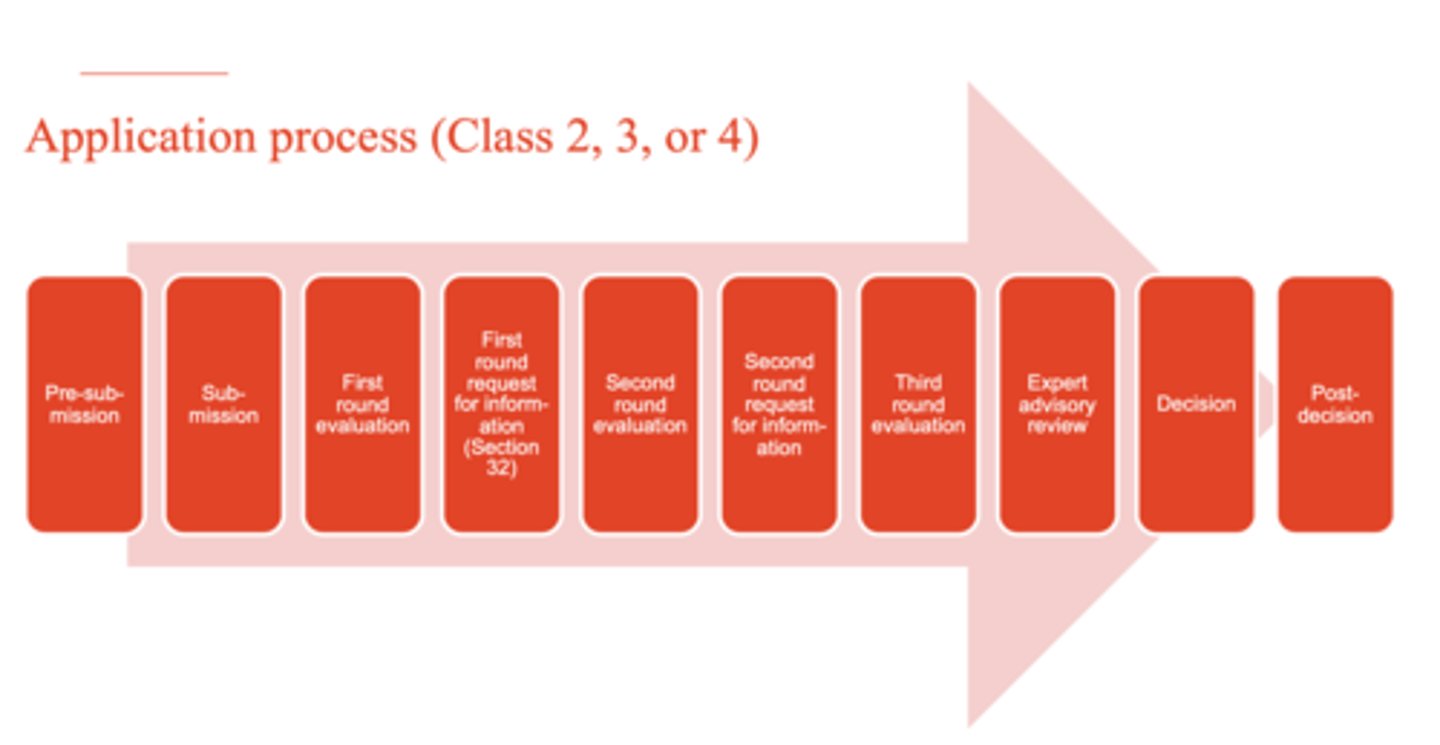
What is the application process for Class 2, 3, or 4 biologicals? (10)
1. pre-submission
2. submission
3. first round evaluation
4. first round request for information (Section 32)
5. second round evaluation
6. second round request for information
7. third round evaluation
8. expert advisory review
9. decision
10. post-decision
Regulatory integration of GMOs
see graph
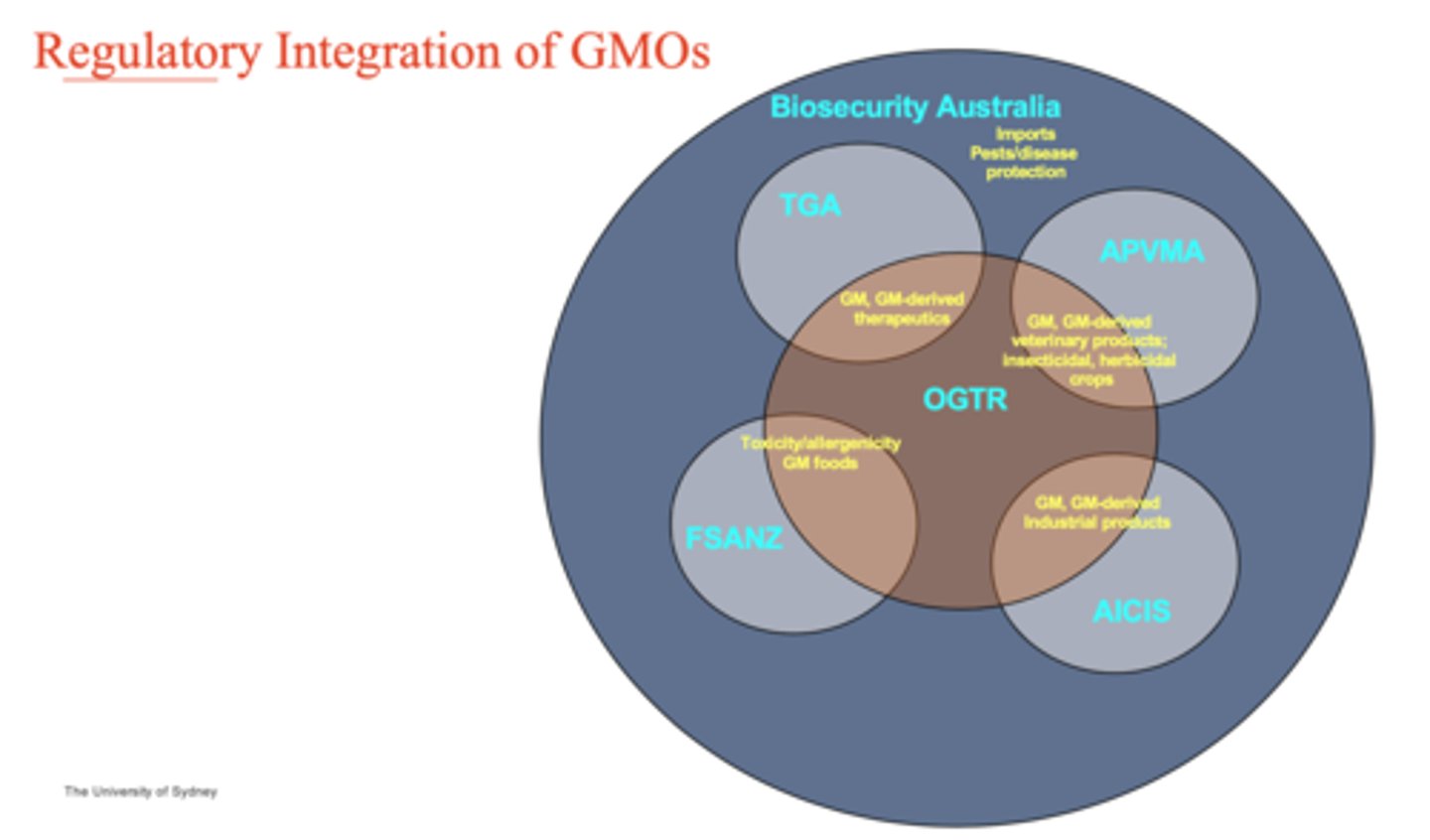
What is the Gene Technology (GT) Act 2000? (6)
- passed in December 2000
- came into effect on 21 June 2001
- a national scheme for the regulation of genetically modified organisms (GMOs)
- legislation mirrored in State/Territory Acts
- GT Regulations 2001 - day to day information on operation of the Act
- User's Guide - plain English aid to interpretation of Act, Regulations
What is a GMO? (4)
- an organism that has been modified by GT
- an organism that has inherited modified traits from a modified organism
- anything declared by the regulations to be a GMO
- does not include humans who have received somatic cell gene therapy or an organism declared by the regulations not to be a GMO
What are the potential dealings associated with GMOs? (6)
- propagate the GMO
- make, develop, produce or manufacture the GMO
- grow, raise or culture
- breed the GMO
- use the GMO in the course of manufacture of a thing that is not a GMO
- importation
What are the GMO authorisations for this dealing? EXEMPT DEALINGS
knockout mice
What are the GMO authorisations needed for notifiable LOW-RISK DEALINGS?
lab-based experiments
What are the GMO authorisations that require a LICENCE but does NOT involve intentional release into the environment (DNIR)?
some gene therapy, manufacturing, vaccines
What are the GMO authorisations that require a LICENCE but DOES INVOLVE intentional release into the environment? (DIR)
GM crops, some gene therapy, vaccines
What are GMO authorisations that require a LICENCE for INADVERTENT DEALINGS
petunia plants
What are the GMO authorisations on the GMO register that NO LONGER REQUIREs a licence and is deemed safe?
blue carnations
What are the GMO authorisations that involve EMERGENCY DEALING DETERMINATION?
equine influenza vaccine --> a few years ago there was an outbreak in Australia and had to be brought in from oversease
What are the delivery methods for gene therapy? (2)
1. Ex-vivo = extract stem or progenitor cells --> deliver targeted nucleases to cells by physical, chemical or viral methods --> introduce modified cells back into patient
2. In vivo = adeno-associated virus (AAV) --> lipid nanoparticle --> direct delivery to patient using viral or non-viral delivery vehicle
How do you know which gene therapies are delivered in vivo or ex vivo? (2)
- ones that have 'vec' at the end of the name are done in vivo (vector) --> PRESCRIPTION TGA
- ones that have a 'cel' at the end of the name are done ex vivo --> BIOLOGICAL TGA
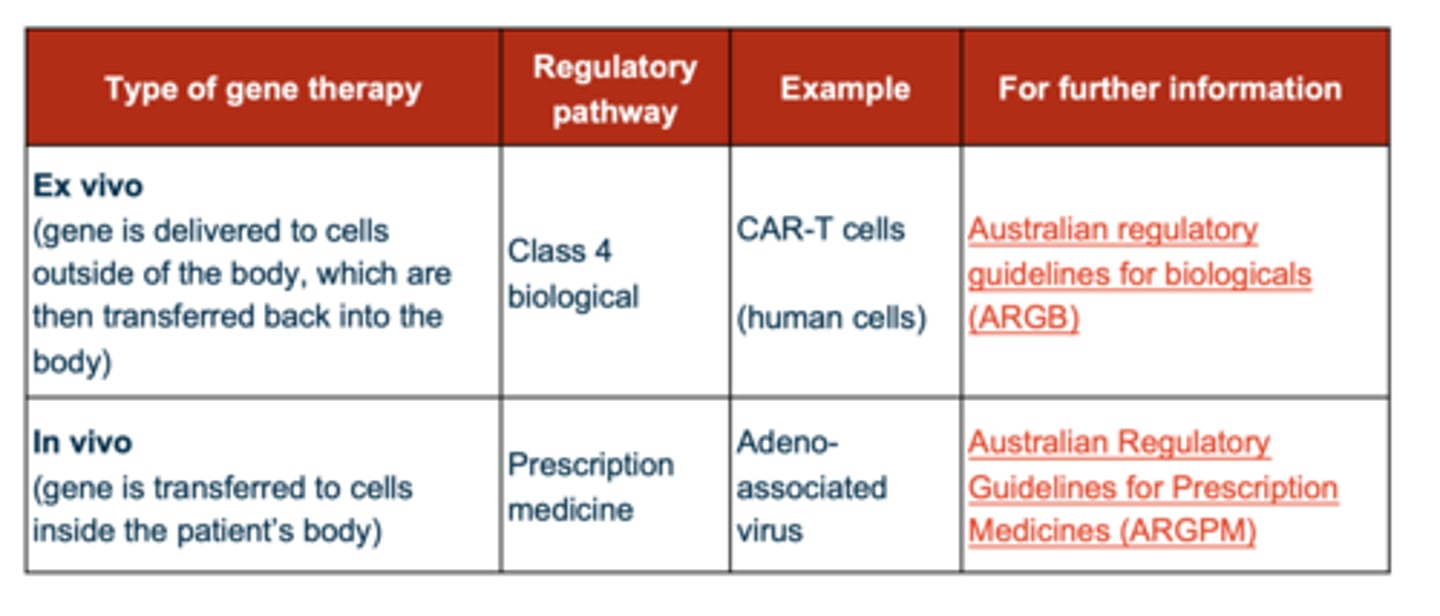
Which of the following need a licence from OGTR? (2)
- in vivo needs a license because they're genetically modified vectors
- ex vivo have been EXCLUDED by the gene technology act
List some examples of Class 4 biological cell therapies?
- Kymriah (tisagenlecleucel)
- Yescarta (axicabtagene ciloleucel)
- Tecartus (brexucabtagene autoleucel)
- Carvykti (ciltacabtagene autoleucel)
List the future trends in therapeutic product development (9)
- precision medicine
- cell therapies
- gene therapies
- new genetic editing techniques
- 3D printing - implants, tablets, tissue reconstruction
- brain-device interfaces
- nanotechnology
- synthetic biology
- AI-driven devices