C19 - Temperature
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
New cards
Temperature
Equal temperature between two objects means thermal equilibrium between them
2
New cards
Celsius
0ºC = ice point
100ºC = steam point
K = C + 273.15
F = 9C/5 + 32
3
New cards
Kelvin
0K = absolute zero
273.15 K = triple point of water
C = K - 273.15
4
New cards
Fahrenheit
32ºF = melting point
100ºF = boiling point
C = (5/9)(F-32)
5
New cards
avg. coeff. of linear expansion
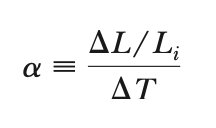
6
New cards
avg. coeff. of volume expansion
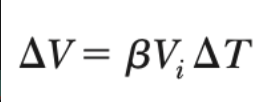
7
New cards
state eqn for ideal gases
PV = nRT = NkbT
R = 8.314 (J)/(mol•K) = 0.0821 (L•atm)/(mol•K)
kb = R/NA = 1.38 × 10-23 J/K
N = # of molecules
8
New cards
Avogadro’s #
NA = 6.022 × 1023 molecules