1/2) Intro / Aqueous geochem and hydrologic cycle
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
How do we quantify water-rock interactions
chemical analysis: concs of ions in water
tracers: evaporation, mixing, dilution, measure isotopes
geochemical models: thermodynamic and kinetic models, quantify the state of a system
can concentration of Ca+ be less than 0 in a natural water
No
can pH be <0? why?
Yes, because pH = -log[H+]
concentration above 1 mol/L will result in negative pH
what elements are present in most or all waters?
what elements are trace pollutants
H, C, O, Na, Mg, K, Ca
Pb, Hg
what are the major cations
what are the major anions
what are they both analyzed by?
Ca2+, Mg2+, Na+, K+
analyzed by ICP-OCT
HCO3-, SO42-, Cl-
HCO3- titration, others analyzed by IC
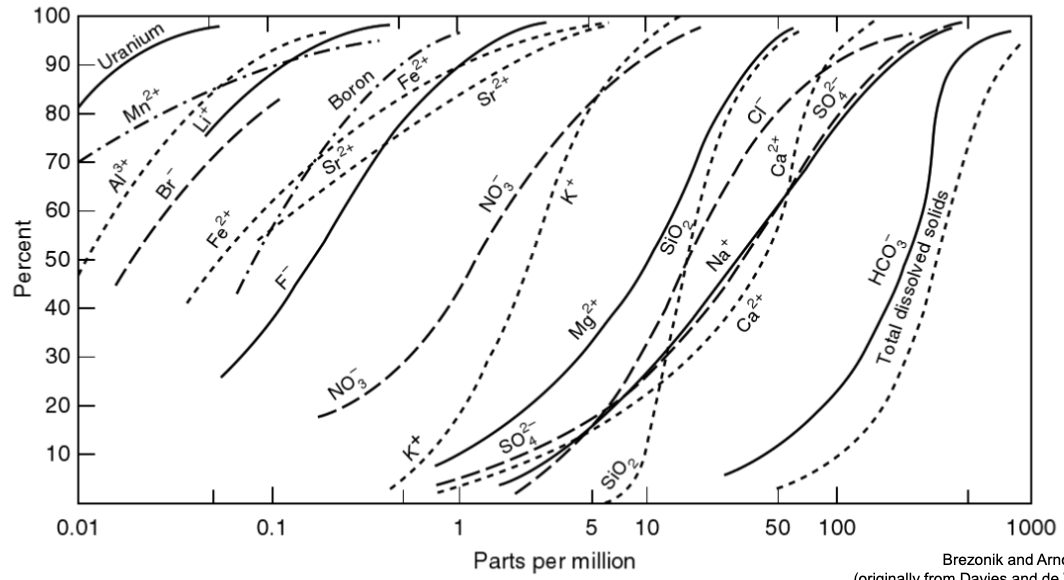
in this graph, what does the steepness of the curves indicate?
details on Silica
details on aluminum
solubility
SiO2, no samples <5, and none >50
Al3+ doesn’t have a control on solubility because a lot of minerals have Al and it affects the minerals in different ways

explain this table
total HCO3- in rivers is 0.96, more than half (0.58) comes from atmosphere
atmosphere is most significant source of SO42- to rivers
comes from coal burning (SO2 gets oxidized to sulphate (SO42-)
which rocks and minerals contribute to the silicate elemental flux?
granite = quartz, plagioclase, kspar = Si, Ca Na, K
basalt = olivine, pyroxene, plagioclase = M,, Ca Na
explain silicate weathering thermostat
thermostat = constant temperature
Ca coming from plagioclase weathers and is transported by rivers, eventually forming carbonates in the ocean, and this process consumes atmospheric CO2
atmospheric CO2 combines with water, turns silicate minerals into calcite and kaolinite
silicate weathering speeds up in hot and slows down in cool temperatures. So when the CO2 is removed from the atmosphere, it cools the atmosphere, and silicate weathering slows down, allowing more CO2 to build up and the temp increases again, increasing the rate of silicate weathering
details on carbonate weathering from table
most of Ca2+ and Mg2+ is coming from carbonates
half of HCO3- comes from atmosphere, so HCO3- ≠ Ca2+ + Mg2+
Ca2+ > Mg2+ because more calcite than dolomite (harder to form)
which minerals are responsible for sulfate-derived flux?
CaSO4
anhydrite or gypsum
these are evaporites from seawater
why are sulfides a source of SO2-
FeS2 + O2 → SO42- + Fe3+
reaction gives no iron in rivers because iron is not soluble in water, it makes FeO or FeOH like hematite, geothite, ferrihydrite
what is the ultimate source of Cl minerals?
evaporites, salts, evaporated seawater
Halite = NaCl
sylvite = KCl
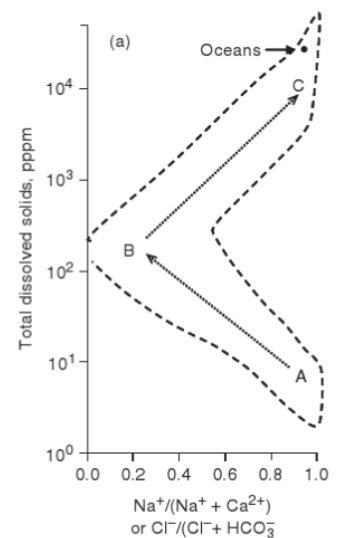
this graph (numbers)
look at axis
Na/(Na+Ca)
if Na is a large fraction of total anion balance, number is bigger, plot right
If Na is a small fraction of total anion balance, number is smaller, plot left
same with Cl/(Cl+HCO3-)
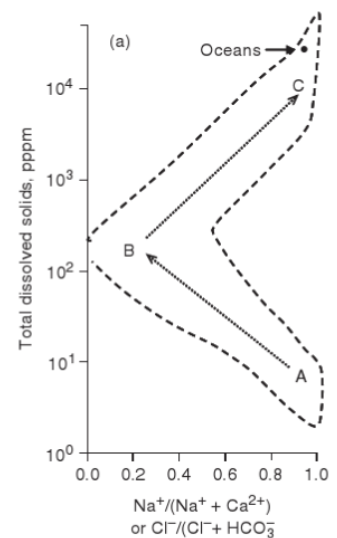
this graph (concepts), which water type each letter
C: oceanwater, lots of dissolved solids and lots Na+ and Cl-
B: groundwater, most groundwaters interact with CaCO3, so they are dominated by Ca+ and HCO3-, hence small number on left
A: rainwater, not a lot of dissolved solids, very dilute but what there is is mostly Na and Cl-, dominated by seaspray
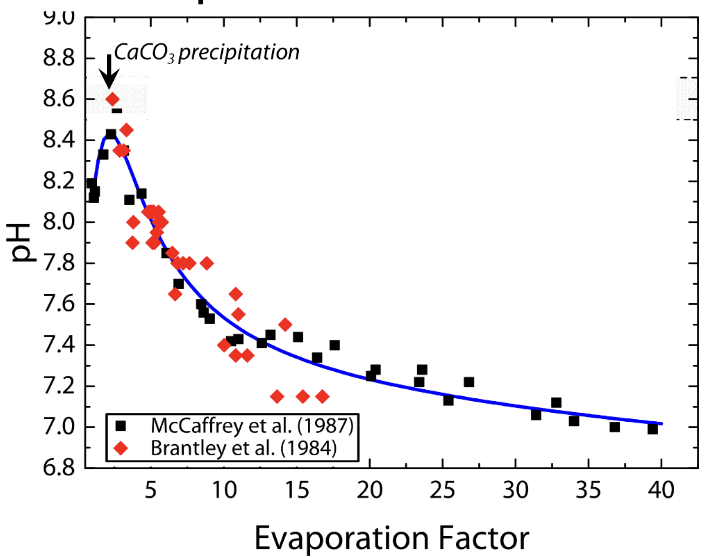
explain this graph
initial water is K/Cl
If K increases at the same rate as Cl, then K is not being precipitated as a mineral
If K increases at a slower rate than Cl, then it’s precipitating out
If K increases at a faster rate, then Cl precipitates instead
what is pH of natural rain
CO2 easily dissolves in water creating H2CO3 (weak acid)
natural pH of rainwater is about 5.6
what causes acid rain
fossil fuels contain reduced sulfur and nitrogen
combustion results in oxidation of these reduced elements (S→SO2, N→NO2-)
info on precipitation chemistry
near the coast, precip looks life seawater
in continental interior, looks like very dilute seawater, some extra Ca2+
near industrial activity, So4 and NO3- are high, pH is low
dust in the air slightly neutralizes pH in the interior
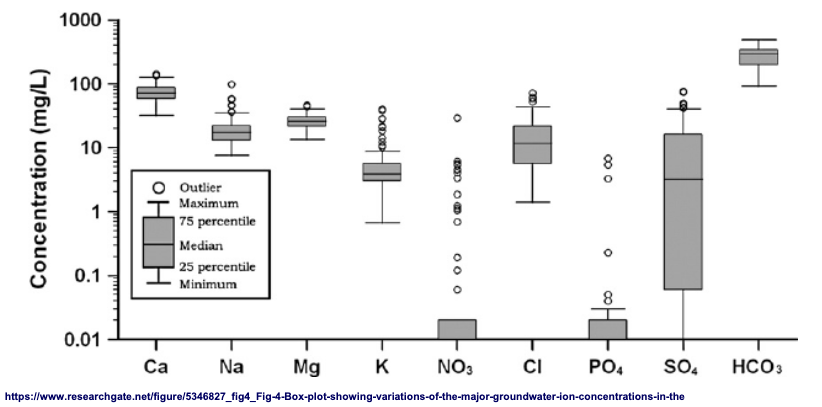
details on chemical distb in GW
Ca is very tightly grouped together, reflects solubility
SO4 has highly variable solubility
is partial pressure of CO2 in GW higher or lower than atmosphere?
higher, from decomposers in the subsurface
log(pCO2) in atm = -3.5
log(pCO2) in GW = -2
negative, higher
evaporation increases ___, traceable by ___ concentrations
increases TDS, by Cl- concentrations
carbonates are generally ___ and ___ in chemistry
more reactive and more reflected in chemistry