24-particle physics, 25-radioactivity
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
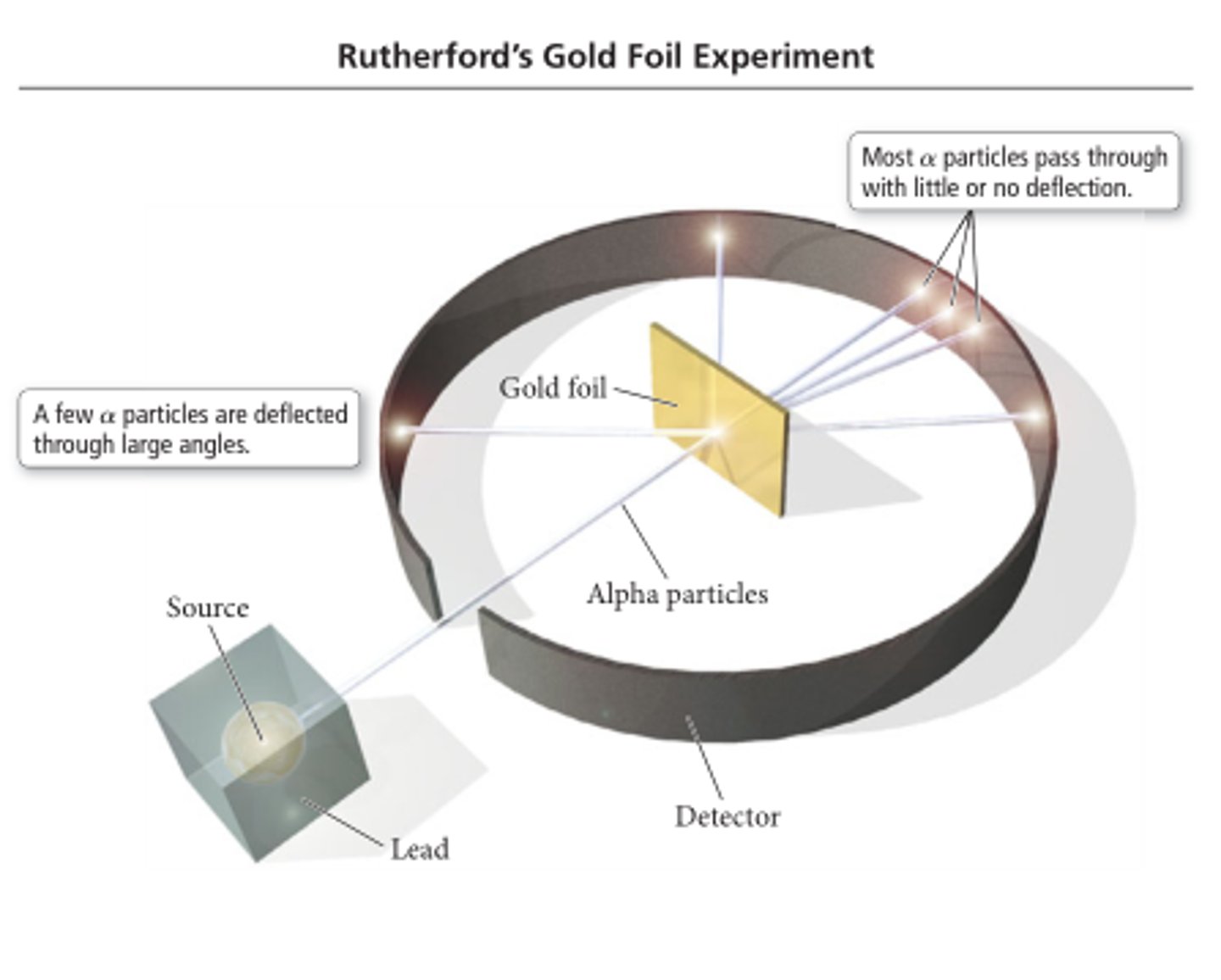
-Explain Rutherford's alpha-particle scattering experiment
-explain the reasons that led to the conclusions for the new standard model of the atom
-alpha particles shot at thin gold foil in a vacuum surrounded by a flurecent screen -> when an alpha particle hit, the creeen produced a tiny speck of light
- most alpha particles went straight through = most of the atom was empty space
- very few were deflected in large angled = small, dense, positive nucleus
- very few were deflected by angles greater than 90º meaning the nucleus is very small and contains most of the mass
- therefore the size of the nucleus is much smaller than the atom
- therefore, electrons must be orbitting the nucleus to give an atom its neutral charge from the positive nucleus
how to calculate the (upper bound) radius of the nucleus by shooting a high energy alpha particle at a nucleus?
what law of conservation allows this method to work?
in the other forumla, what does each of the values represent?
- using a high energy alpha particle, we can calculate the upper bound for the radius of the nucleus
- the distance 'd' of closest approach to the gold nucleus can be calculated using the idea of conservation of energy:
-at this distance, the alpha particle momentarily stops before being deflected back meaning the initial kinetic energy is completely converted into electrical potential energy
- we can use ½mv² = Qq/4πεºd (Coulomb's law)
where Q and q are the alpha particle and nucleus, ε is the permittivity of free space
- rearrangd for d gives us the upper bound of the radius of the nucleus
What is a nucleon?
a proton or neutron in a nucleus
how is the nucleus of the atom represented?
what makes somehting an isotope?
- represented as shown where A is the nucleon number (mass number), Z is the proton number (atomic number) and X is the element
- isotopes of the same nuclei have the same number of protons but different number of nuetrons
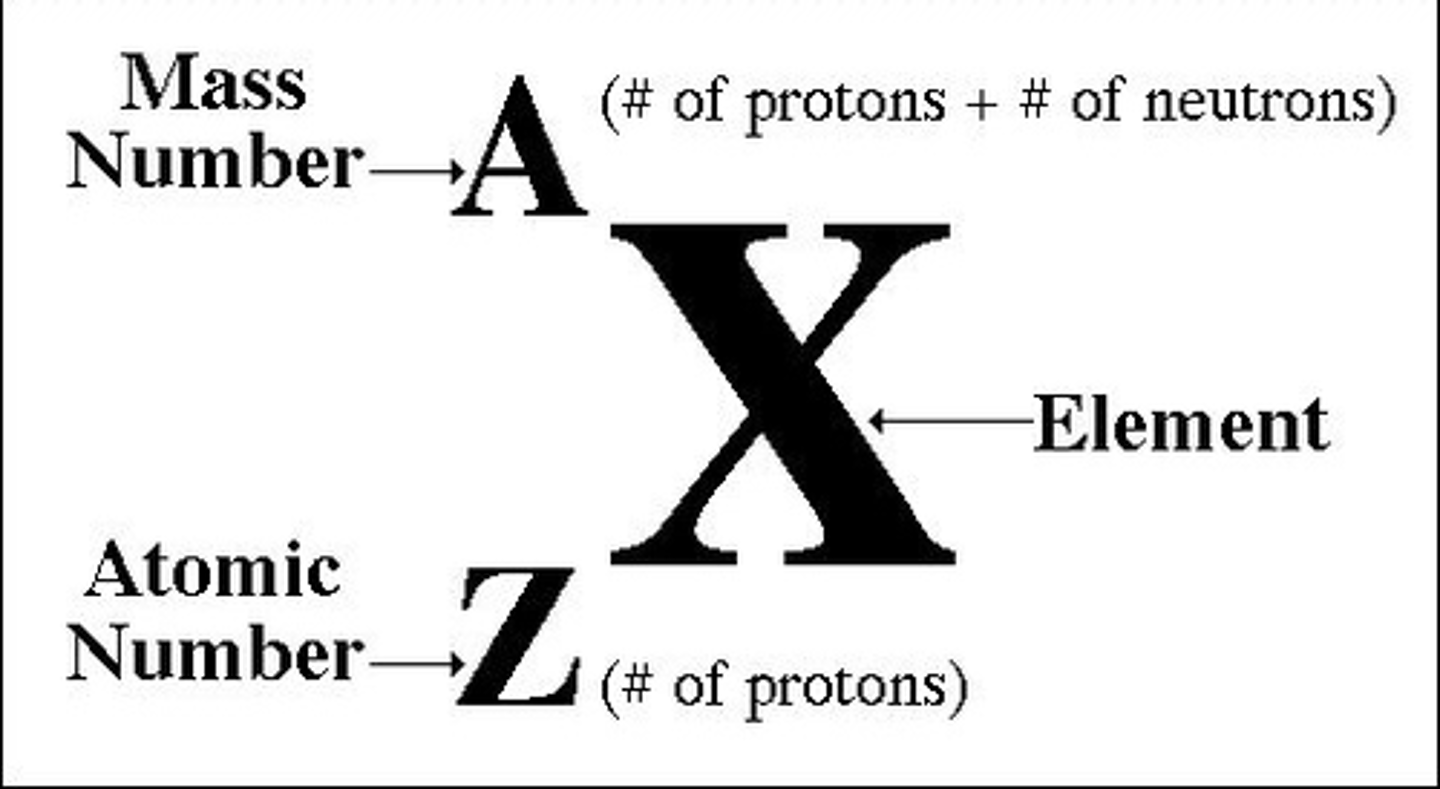
what is the atomic mass unit?
one twelfth the mass of a carbon-12 atom
∴ 1u = 1.1661x10^-12
how to caclulate the density of a nucleus?
what is the value of the constant?
what is A ?
- to calculate the density of a nucleus -> ρ = m/v
- v = πr^3
- R = rºA^⅓
- rº = 1.2 fm, A = nucleon number
- m = 1u x A
∴ can solve for density or volume or mass
-what does the gravitational force affect, strong or weak, what is its range?
-what is the electromagnetic force comprised of, what particles does it act on, what is its range?
-what the weak nuclear force responsible for, what does it do to quarks?
- what does the strong nuclear force do, what force does it counteract, between what range is it attractive and repulsive?
- the gravitational force is an attractive force that has infinite range but very weak and only acts on particles with mass
- the electromagnetic force is comprised of electrostatic and magnetic forces with an infinte range and acts on particles with charge
- the weak nuclear force is responsible for beta decay. it acts to change quarks over very small distances
- the strong nuclear force acts between all nucleons and all quarks and counteracts the repulsive electrostatic forces between protons in the nucleus. it is attractive to 3 fm and repulsive below 0.5 fm
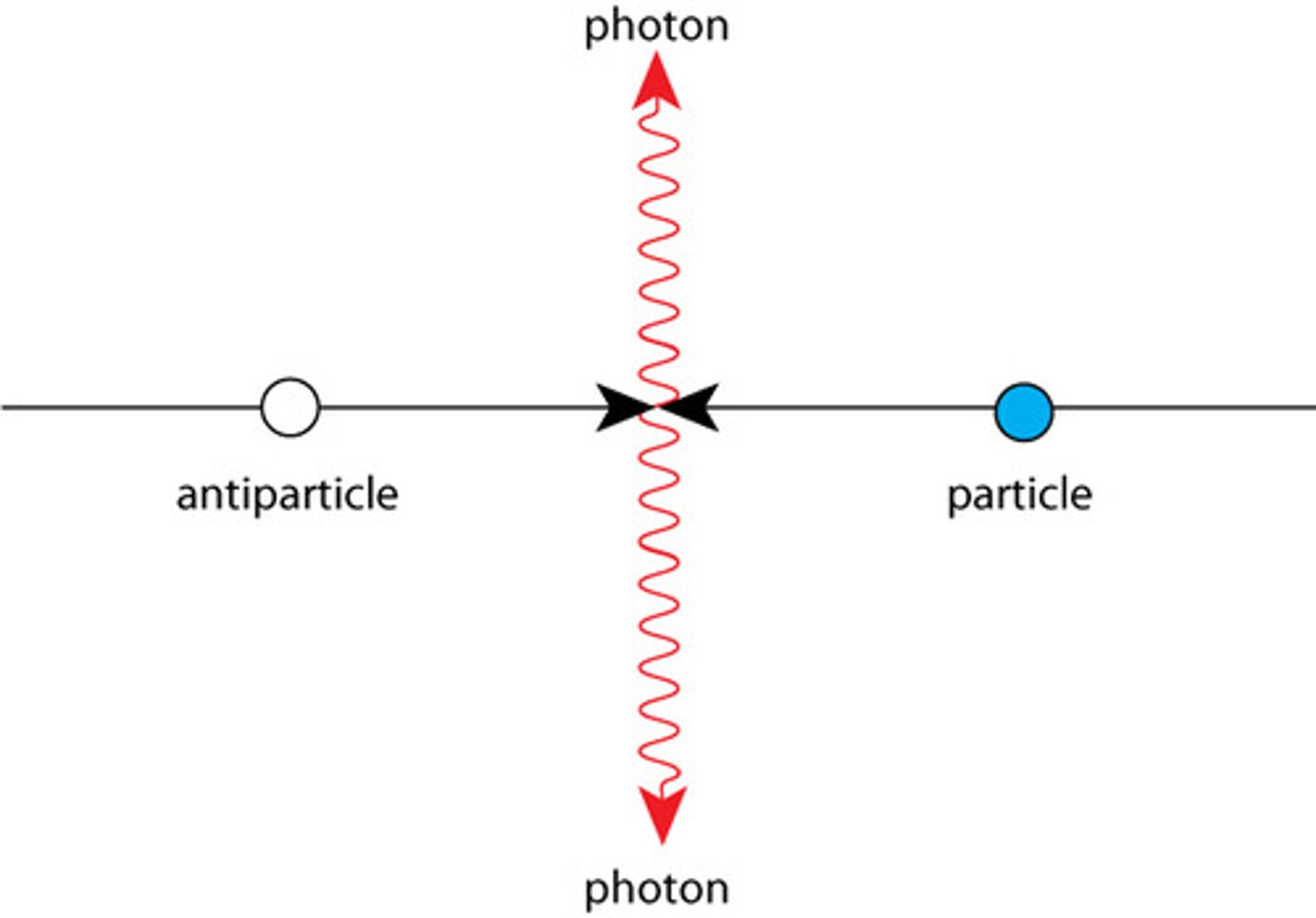
what is a simliarity and a difference between a particle and its antiparticle?
describe the process of annhiliation in terms of mass and energy?
how do you calculate the total energy released of what is produced ?
- a particle and its antiparticle will have the same mass and opposite charges
- when an antiparticle and a particle meet, they completely destroy each other in a process called annihilation
- annihilation is where total rest mass energies and any kinetic energy of particle and antiparticle are converted into energy in the form of high-energy photons (usually gamma rays) after they meet
- energy released by E = mc²
-their total energy is equal to the rest mass energies + any kinetic energy
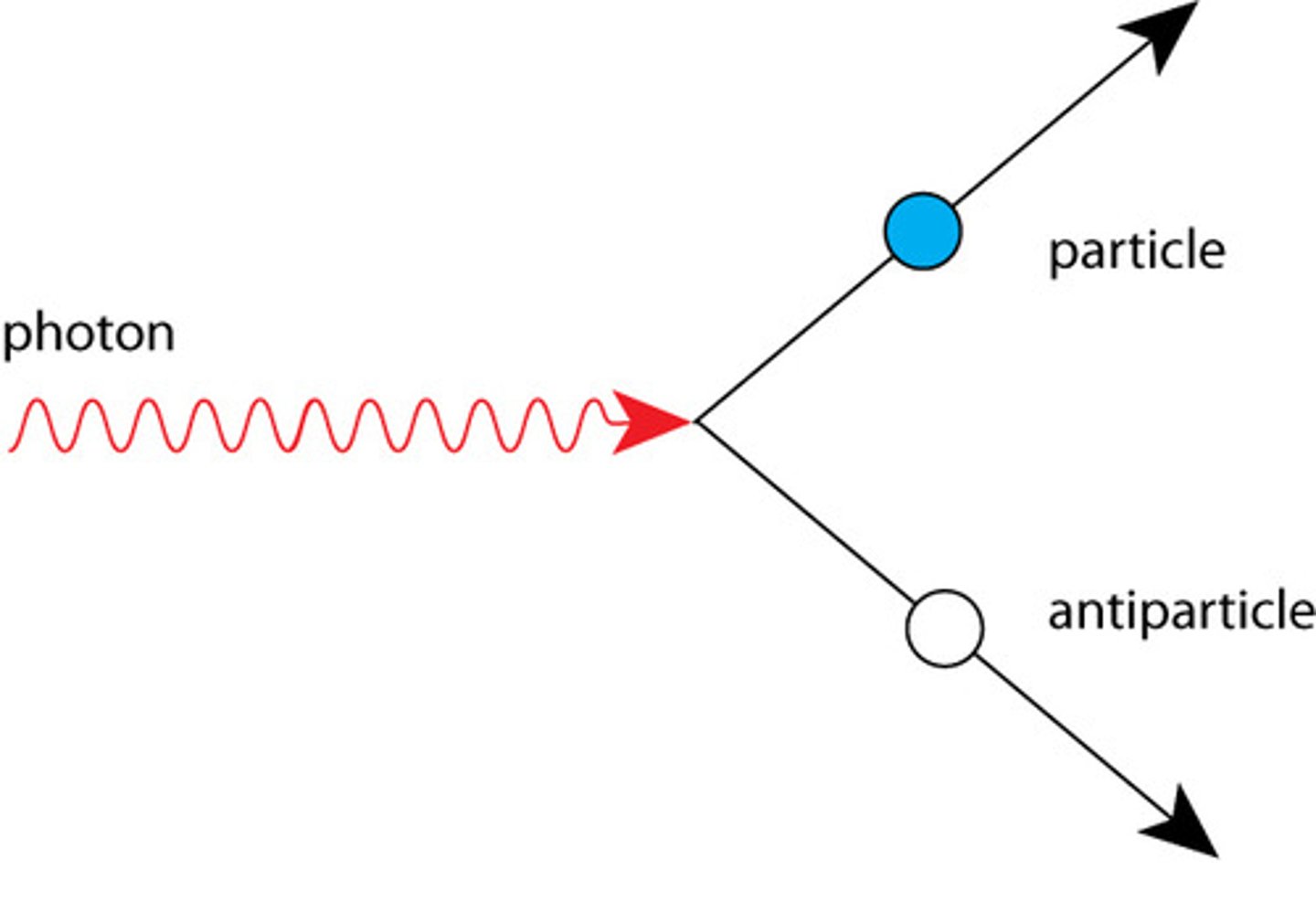
what happens during pair-production?
how much energy is required?
what is the role of the nucleus?
- pair production occurs when a high energy photon is spontaneously converted to a particle anti-particle pair in the presence of a nucleus to conserve momentum
- the photon must have sufficient energy: minimum energy must be equal to the combined rest mass energy of the partcile and antiparticle
- the nucleus also absors some momentum to ensure the conservation of momentum
break down the particle into its fundamental groups and fundamental parts
particle -> hadrons & leptons
- leptons = fundamental = electron, muon, tau. each has a corresponding neutrino (electron nuetrino etc)
- hadrons are composed of quarks. can be composed into subroups; baryons and mesons.
- baryons are made of 3 quarks (proton UUD, neutron UDD). mesons are composed of a quark and an antiquark (U antiD), examples are pions (π) and kaons (K)
what forces interact with hadrons?
what about leptons?
- hadrons are affected by the strong-nuclear force and decay by the weak nuclear force
- leptons are not affected by the strong-nuclear force but subject to the weak nuclear force
-both if charged interact with electromagnetic force
Charge of up, down and strange quark? and the corrosponding antiparticles
quarks;
up = +⅔e , anti-up = -⅔e
down = -⅓e, anti-down = +⅓e
strange = -⅓3e, anti-strange = +⅓e
what happens during beta-minus decay?
In terms of forces, quarks and emitted energy, explain the process of beta-minus decay
-a neutron in an unstable nucleus decays into a proton, an electron and an electron antineutrino
- in beta-minus decay, the weak force mutates a down quark into an up quark within a neutron, which transforms it into a proton. this process emmits a W-boson which quickly decays into an electron and antineutrino to conserve charge and lepton number.
what happens in beta-plus decay?
In terms of forces, quarks and emitted energy, explain the process of beta-plus decay
in beta-plus decay, a proton decays into a neutron, a positron and an electron neutrino
-due to the weak nuclear force, an up quark mutates to a down quark in the proton, transforming it to neutron and emits energy in the form a positron and an electron neutrino.
what is the nature of alpha radiation?
what is the nature of beta radiation?
what is the nature of gamma radiation?
(comprised of? charge? penetration power? ionising power? range? speed? wavelength? stopped by?)
Alpha radiation - positively charged alpha particles comprised of 2 protons and two neutrons (+2e) + highly ionising + low penetration power: few cm of air or thin paper + low range: few cm in air
Beta radiation - fast-moving electrons (β-) or positrons (β+) + moderate penetration power: few cm of aluminium + lower ionising power + range: few metres in air + speed: close to the speed of light
gamma radiation (rays) - high-energy photons with λ<10^-13m + high penetration power: stopped by lead+ low ionisation + unlimted range + travelling at the speed of light + no charge
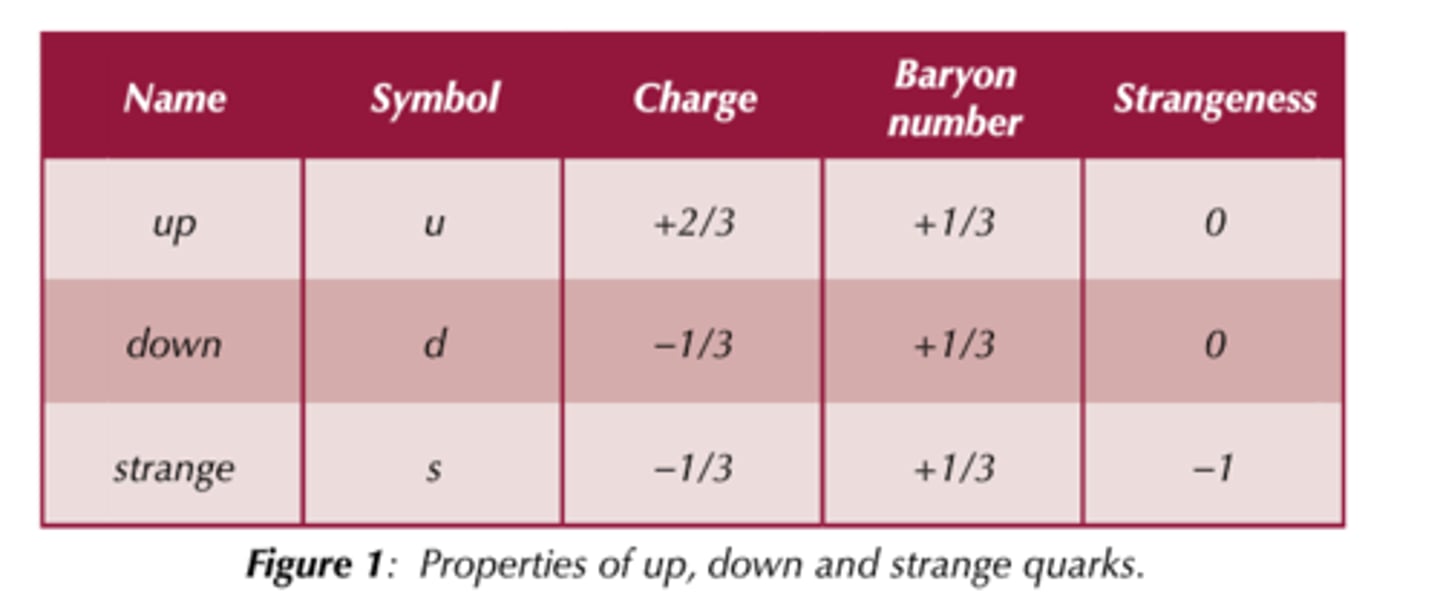
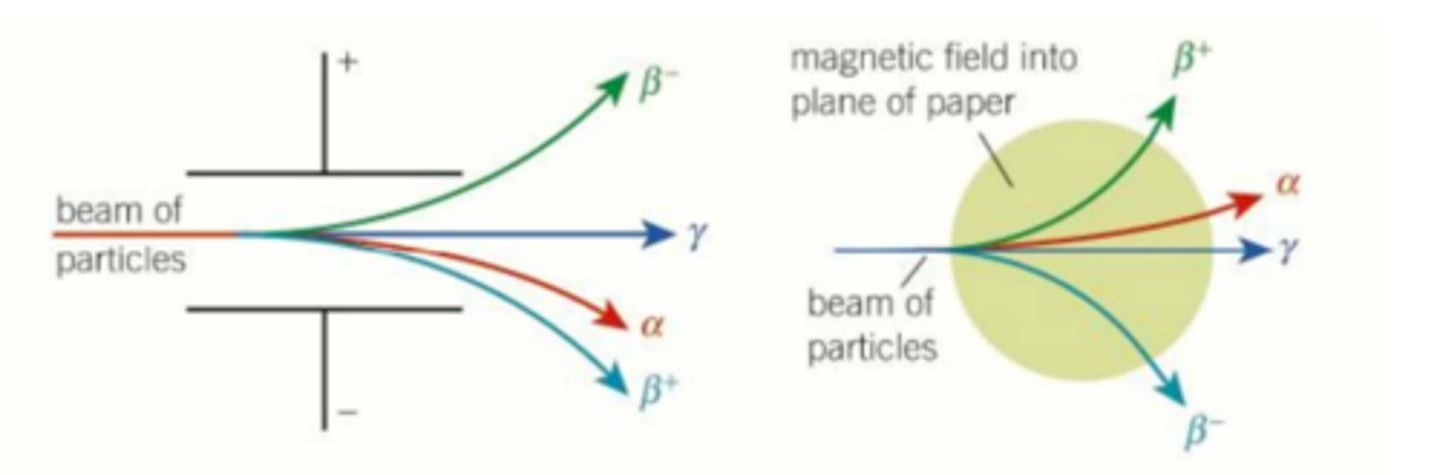
how can an electric field be set up and used to distinguish between different types of radiation?
A uniform electric field, provided by 2 oppositely charged parallel plates, can distinguish between different types of radiation due to their charges and their mass (charge deflects to oppositely charged plate, lower mass means higher gradient of deflection)
what is the danger of radioactivity?
what would you store a source in to protect from radioactivity?
how would you transfer a radioactive source from one place to another to protect from dangers?
dangers of radioactivity:
- ionisation can damage living cells ∴ store radioactive sources in a lead-lined container
- when transferring radioactive sources, use a pair of tongs with long handles to keep away from your body
explain the alpha decay equation
what causes a nuclei to emit β+ or β- ?
when does gamma decay take place?
alpha decay - parent nucleus decays into daughter nucleus (different element) + energy is also released
beta decay - caused by weak nuclear force
β- : nuclei has too many neutrons for stability
β+ : nuclei has too many protons for stability
gamma decay - gamma photons are emitted of a nucleus has surplus energy following an alpha or beta emission
Define radioactive decay
what are the key points making it random and spontaneous?
radioactive decay is the random and spontaneous breakdown of an atomic nucleus resulting in the release of energy and matter from the nucleus
random: cannot predict when a particular nucleus will decay + each nucleus within a sample has the same chance of decaying per unit time
spontaneous: decay of nuclei is not affected by presence of other nuclei or external factors like pressure
define the half-life of a source
what does the half-life of a source depend on?
half-life:
- the probability of decay governs how quickly the nuclei decay and, therefore, the half-life of the isotope
- the half-life, the average time taken for half the number of active nuclei in the sample to decay
define activity (A) of a source
what is it measured in?
what does activity depend on?
- the activity A of a source is the rate at which nuclei decay
- measures in decays per second (Becqurerel = Bq)
- activity depends on the half-life and the number of undecayed active nuclei N
define the decay constant
The probability of decay of an individual nucleus per unit time