BIOCH 200: Slide 8 (Oxidative phosphorylation)
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
Catabolic pathways…
a. Oxidize metabolites
b. Generate reduced cofactors
c. Both A + B
d. Neither A nor B
C
What 2 purposes do catabolic pathways serve?
Break down large molecules into BUILDING UNITS
Release and temporary storage of E in High E molecules
ATP/NTPs
Reduced cofactors (NADH/FADH2)
Are catabolic pathways oxidative or reductive? What happens to metabolites + cofactors.
Oxidative
metabolites = oxidized
Cofactors = reduced
What does the Re-oxidation of cofactors after they are reduced do?
Generate ATP
What 2 processes is oxidative phosphorylation made up of?
Oxidation of reduced cofactors (NADH/FADH2) by the reduction of O2
Phosphorylation of ADP —> ATP
Where does oxidative phosphorylation occur?
A proton gradient across the INNER Mitochondrial membrane
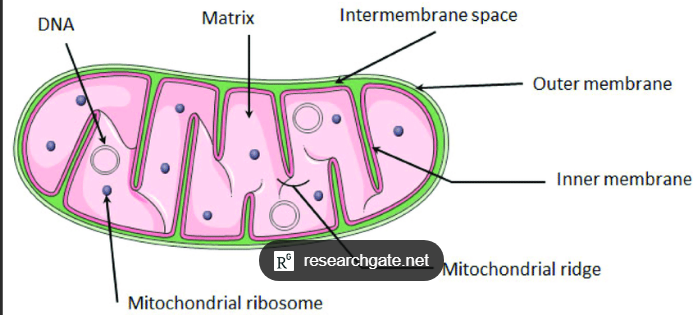
What is the overall flow to generate ATP?
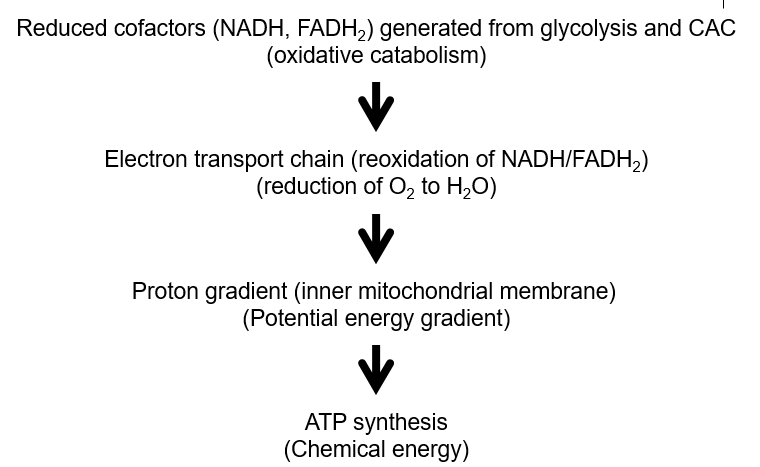
What are the 6 major components of the ETC?
Complex 1, 2, 3 + 4 (integral membrane proteins)
Coenzyme Q (lipid soluble coenzyme)
Cytochrome c (peripheral membrane protein)
What are 4 four electron-carrying cofactors that play a role in re-oxidation of FADH2 and NADH in the ETC
FMN
Iron-sulfur clusters
Cytochromes
Coenzyme Q
How many e- does FMN carry?
2
How many e- do Iron -sulfur clusters carry/pass?
1
What switches between oxidized and reduced sates in cytochromes?/ what takes part in the passing of e- in the ETC?
HEME
cytochromes = hemoproteins
How many e- do cytochromes pass?
1
Fe3+ + 1e- —> Fe2+
What type of molecule is Coenzyme Q?
Lipid soluble molecule
What does Coenzyme Q do?
Transports e- from Complex 1 and 2 —> Complex 3
becomes Ubiquinol QH2
How many e- does Coenzyme Q carry/transport?
2
Q (ubiquinone) + 2e- + 2H+ —> QH2 (ubiquinol)
What is reduction potential?
affinity for e-
What does a higher reduction potential mean?
More negative delta G
True or False: e- move from compounds with higher reduction potential to those with lower reduction potential
False:
e- move from compounds with lower reduction potential to those with higher reduction potential
low reduction potential = don’t want e- that much = willing to give up e-
High reduction potential = really wants e-
Why do e- move spontaneously through the ETC?
e- are passed from components with lower Reduction potential to components with higher reduction potential
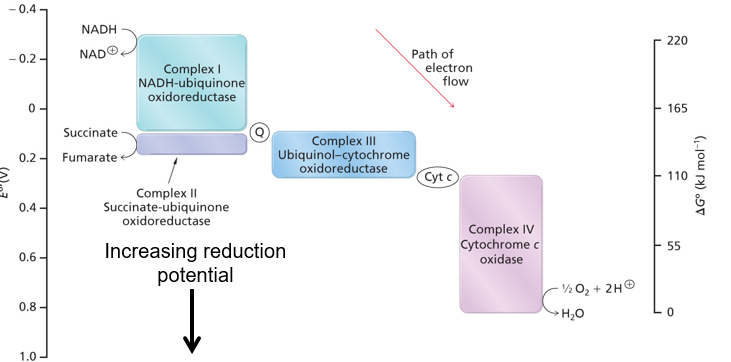
What is generated by the redox reactions of the ETC (low —> high reduction potential)
Free energy
How can the Free E from redox reactions of the ETC be used?
TRANSPORT H+ across the membrane
What type of transport is using Free E of redox reactions to transport H+?
PRIMARY active transport
How does this Primary active transport work in the ETC?
Electron transport causes CONFORMATIONAL CHANGE which allows the complexes to pump H+
Which complexes of the ETC can pump H+? How many are pumped at each?
Complex 1 = 4 (Oxidation of NADH = high E)
Complex 3 = 4 (Q cycle)
Complex 4 = 2 (Reduction of O2 = High E)
How many H+ are pumped out as a result of NADH deoxidization? what about FADH?
NADH = 10
FADH = 6
How many e- are transported from NADH?
2
How many e- are transported from FADH2
2
What is the terminal e- acceptor of the ETC?
O2
reduction of O2 = lots of E
Very high reduction potential
Illustrate the Path of e- from NADH in ETC
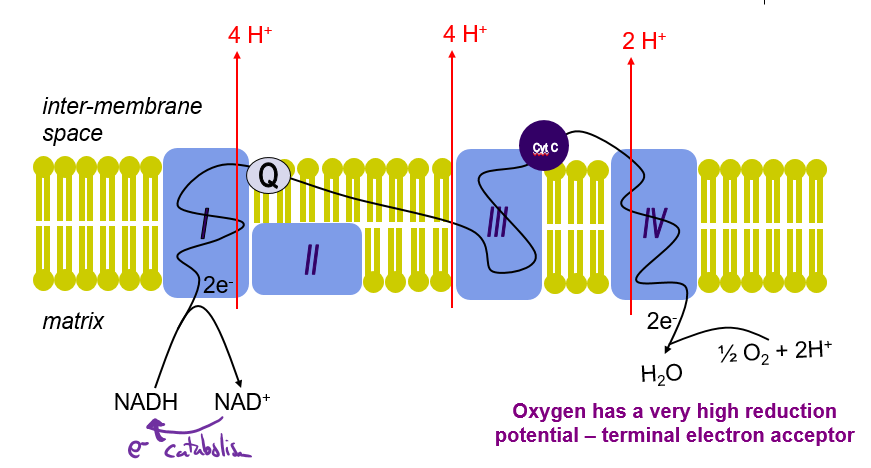
Illustrate the Path of e- from FADH2 in ETC
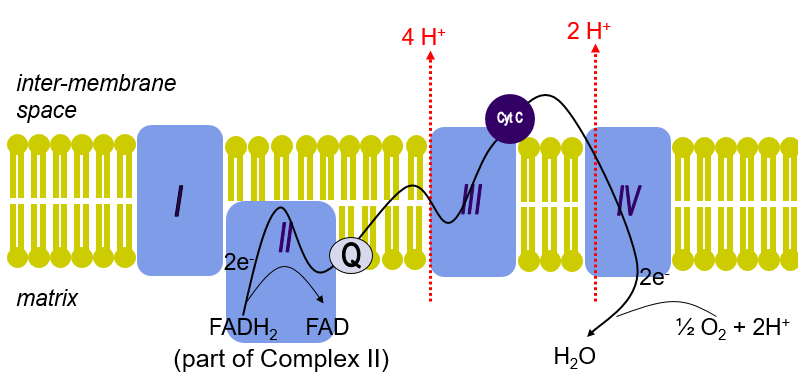
What is complex 2 also known as? Where else can you find it?
Succinate Dehydrogenase
part of CAC
What prosthetic group does Complex 2 contain?
FAD
What does Complex 2 do as succinate dehydrogenase?
Catalyzes oxidation of Succinate —> Fumarate as part of CAC
Where do the e- from the oxidation of Succinate go?
To coenzyme Q in the membrane

What determines the Rate of O2 consumption?
B
A and C both refer to thermodynamics not kinetics
Affinity = thermodynamics
Speed / rate = Kinetics
What drives ATP synthesis by ATP synthase?
the proton electrochemical gradient
What is the proton electrochemical gradient?
PROTON MOTIVE FORCE
difference in proton (H⁺) concentration and charge across the inner mitochondrial membrane
How is ATP formed by the proton electrochemical gradient?
The potential E of H+ gradient = converted to chemical E in the Phosphoanhydride bonds of ATP
Where is the [H+] high and where is it low?
High = Inter-membrane space (b/c ETC complexes)
Low = Mitochondrial matrix
What are the 2 parts of the ATP synthase?
Fo and F1
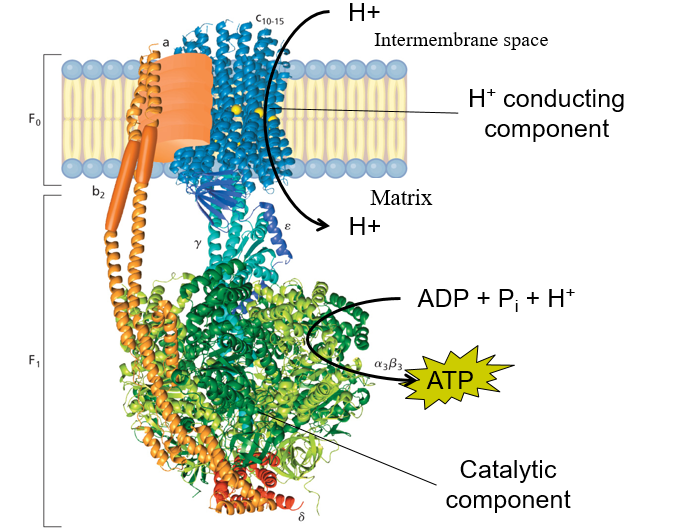
What does Fo and F1 do?
F0 (Transmembrane protein)= Triggers CONFORMATIONAL CHANGE of F1 when protons pass through
F1 = Catalytic portion. Synthesizes ATP from ADP
What does the O of Fo stand for? (don’t know how important)
O = Oligomycin
Oligomycin = inhibits the action of Fo portion of ATP synthase
What determines the rate of O2 consumption?
The rate of ATP synthesis = determines how many [H+] is needed thus ultimately O2 consumption as the Final e- acceptor
How many H+ are needed per ATP by ATP synthase?
3
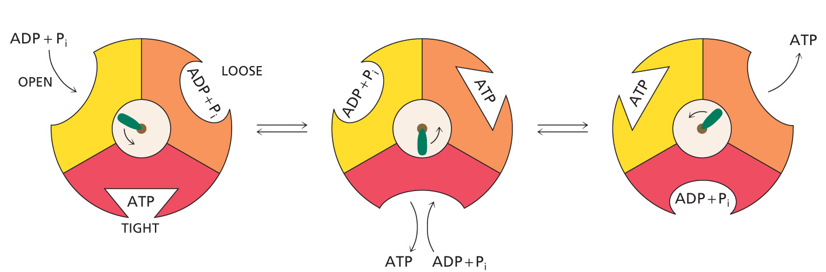
How many ATP are generated by every complete turn of the central shaft of ATP synthase
3 ATP
3 active sites make ATP simultaneously
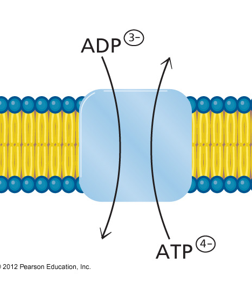
Adenine Nucleotide Translocase?
ANTIPORTER
transports newly synth ATP —> cytosol
Transports ADP (after ATP used) —> matrix
Pi-H+ Symporter?
It transports Pi into matrix for the synthesis of ATP by ATP synthase
Powered by PMF (H+ gradient)
What is the diff in charge and pH inside vs outside the mitochondrial matrix
In = - charge + High pH
Out = + charge + Lower pH
all bc of H+ gradient
Overall what is the Net # of H+ needed to generate 1 ATP?
4
3 for ATP synthase
1 for Pi-H+ symporter
What does it mean by oxidation and phosphorylation are coupled?
The rate of O2 consumption is connected to the rate of ATP synthesis
NADH reoxidation, ETC + O2 consumption = coupled to Rate of Consumption + synthesis of ATP through the Magnitude of H+ electrochemical gradient
What drives the rate of ATP synthesis
the availability of ADP and Pi
What is the P:O ratio?
Amount of ATP made (P: phosphorylation) per O2 atom reduced to water (O)
How many H2O = made per each NADH/FADH2
1
2e- from NADH/FADH2
2H+ + 2e- + 1/2O2 —> H2O
What is the P:O ratio for each NADH reoxidized? and why?
~ 2.5
NADH = 10 H+
4 H+ = 1ATP
10/4 = 2.5
What is the P:O ratio for FADH2?and why?
1.5
NADH = 10 H+
4 H+ = 1ATP
6/4 = 2.5
True or false: The rate of oxidative phosphorylation = determined largely by the relative [ADP]
TRUE
if no ADP = No ATP can be made by ATP synthase = can’t dissipate gradient = gradient doesn’t need to be replenished by the ETC
How does O2 consumption react in response to Increased ADP
consumption increases
What does [ADP] reflect?
The E consumption of a cell
How does the activity of ATP synthase effect the rate of CAC in tissue?
High ATP synthase activity = HIGH NADH/FADH2 usage = low [ ] = activate CAC
Low ATP synthase activity = High [NADH/FADH2] accumulate = Inhibit CAC
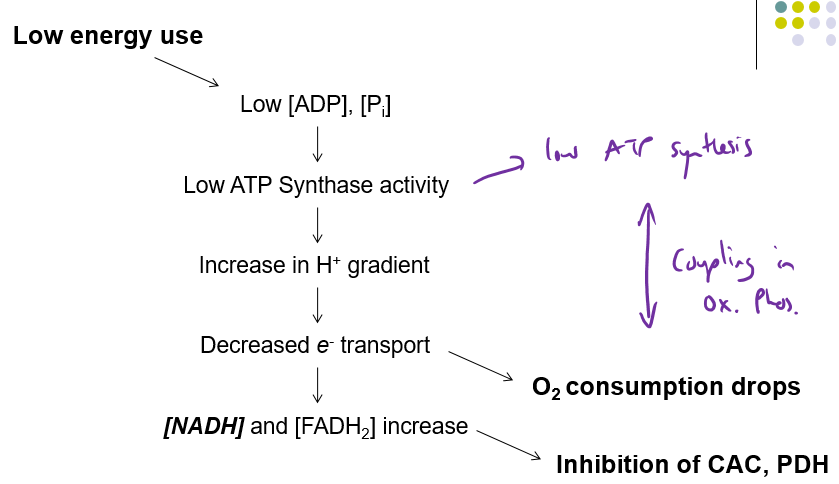
How does Low Energy use affect Coupling of oxidative phosphorylation
PDH = pyruvate dehydrogenase
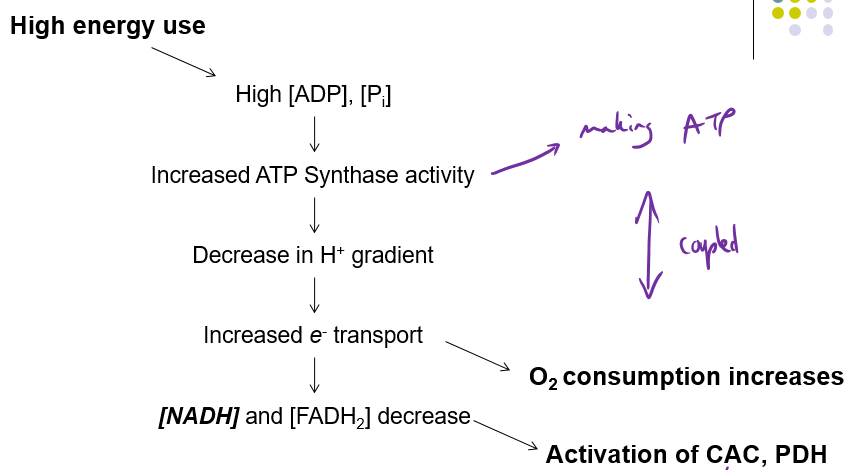
How does High Energy use affect Coupling of oxidative phosphorylation
How does O2 concentration react to the addition of ADP
They decrease
ADP addition = ATP synthase stimulated = O2 Consumption = decrease [O2]
What is an uncoupler/ Uncoupling protein? + how does it affect the system?
Uncoupler = Allows H+ into matrix without making ATP = Uncoupled system
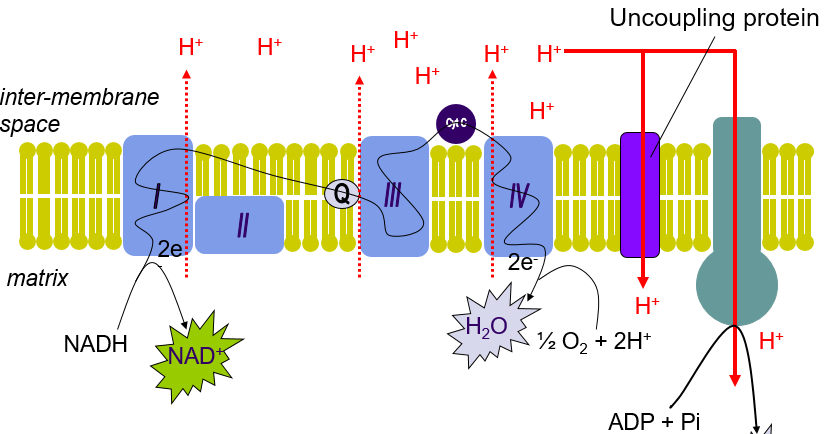
What do uncoupling proteins generate instead of ATP?
HEAT
What is Brown adipose tissue?
Adipose tissue with lots of MITOCHONDRIA it produces heat by using uncoupling proteins to generate heat
What happens to O2 consumption in the presence of an Uncoupler?
O2 consumption increases
Why does O2 consumption increase in the presence of an uncoupler?
Uncoupler = transport H+ without generating ATP (heat instead)
Proton gradient = dissipated faster = ETC rate increases
How do uncouplers affect CAC?
Uncoupler = increase rate of ETC = increase rate of NADH/FADH2 oxidation/usage = low [NADH/FADH2] = Activate CAC

a. Increase
b. Decrease
c. Depends on if NADH/FADH2 = being oxidized
d. no change
B: Less ATP per O2 reduced
Effect of Uncoupler on O2 consumption + ATP prod
2,4-dintrophenol = diet pill (uncoupler)
dissipates H+ gradient
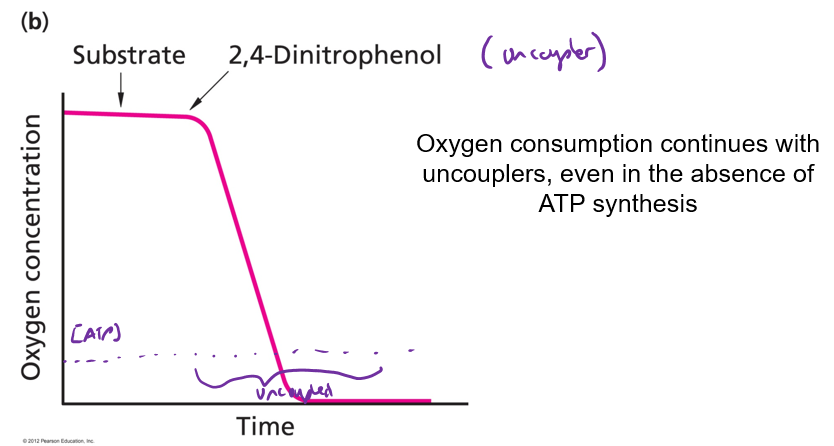
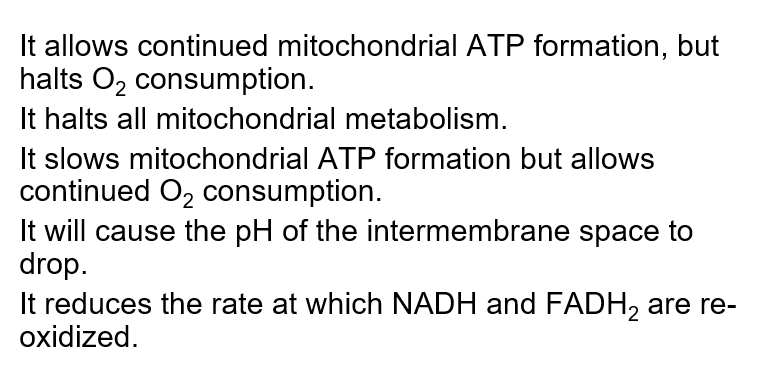
What is the potential consequence of uncoupling mitochondrial oxidative phosphorylation?
C
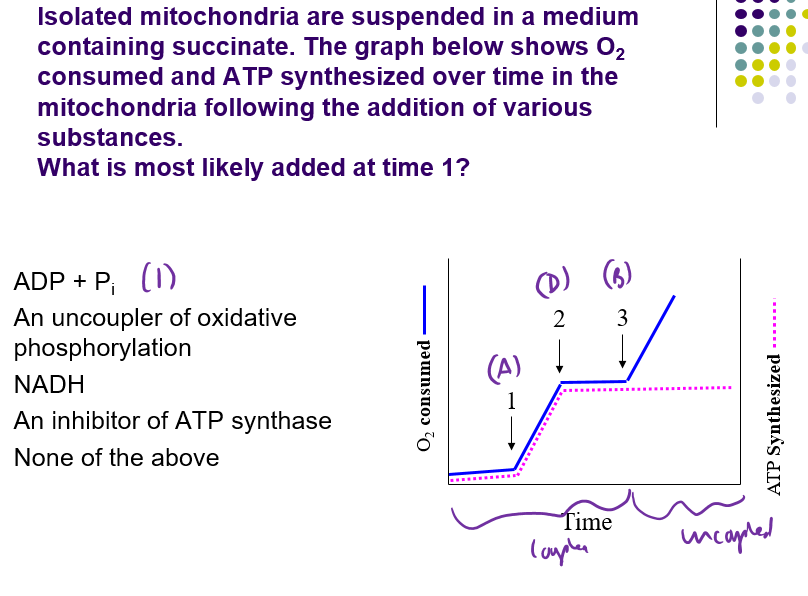
A.
Time 2 = ATP synthase inhibitor
Time 3 = Uncoupler