W4L2: Reactions of alkenes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Reactions and uses of alkenes
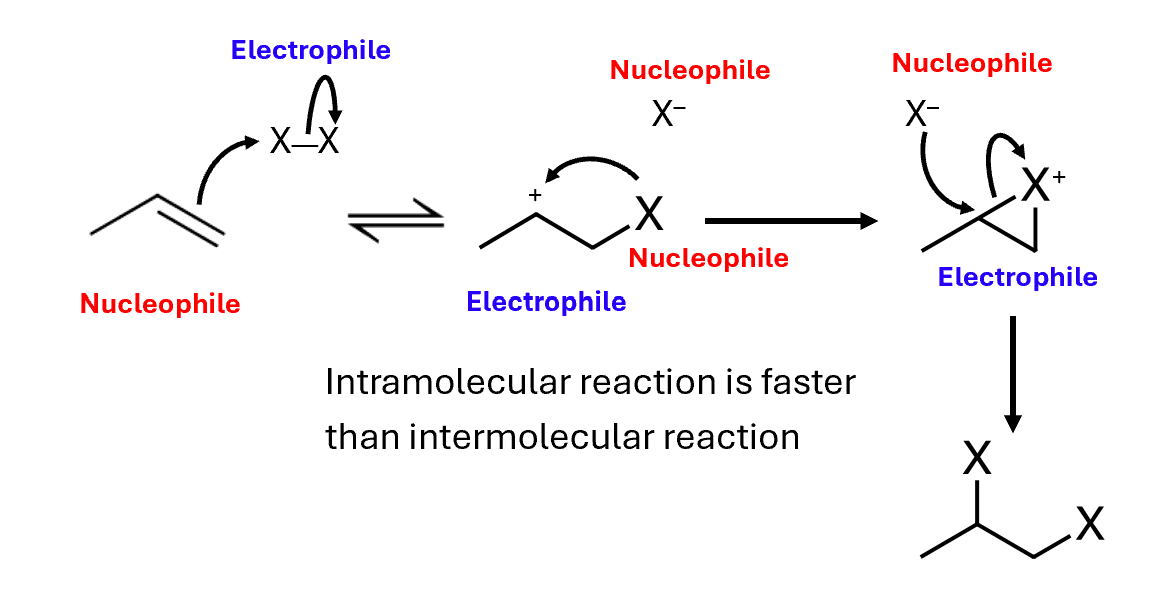
The double bonds of alkenes are much more reactive than the single bonds of alkanes
Free radical oxidation (they also burn)
Hydrogenation (addition of hydrogen) to form alkanes
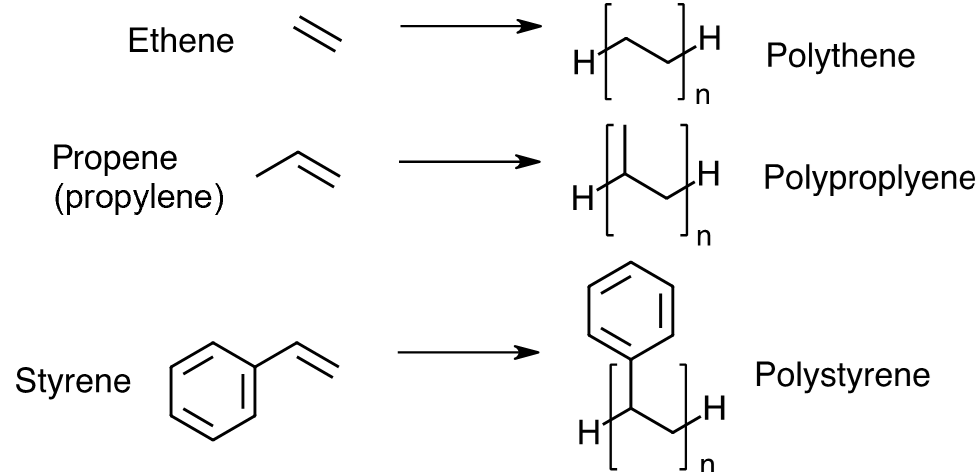
Polymerisation
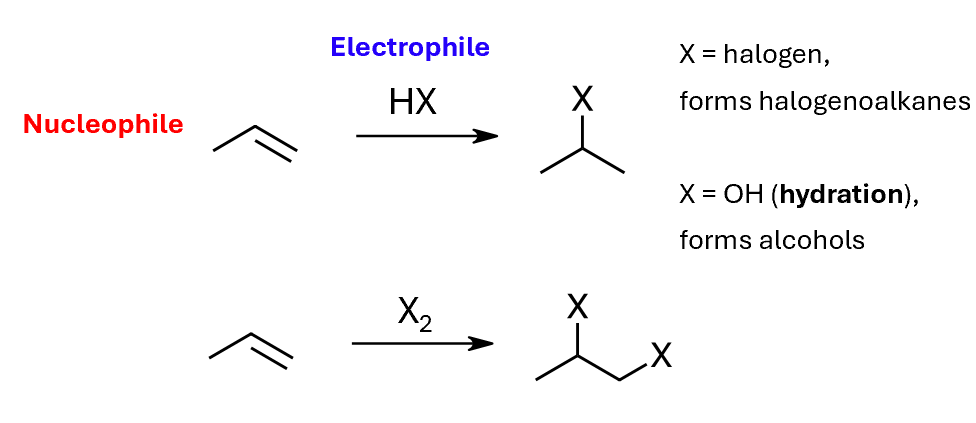
Electrophilic addition reactions, such as with halogens or water
Polymerisation
Electrophilic addition reactions, such as with halogens or water
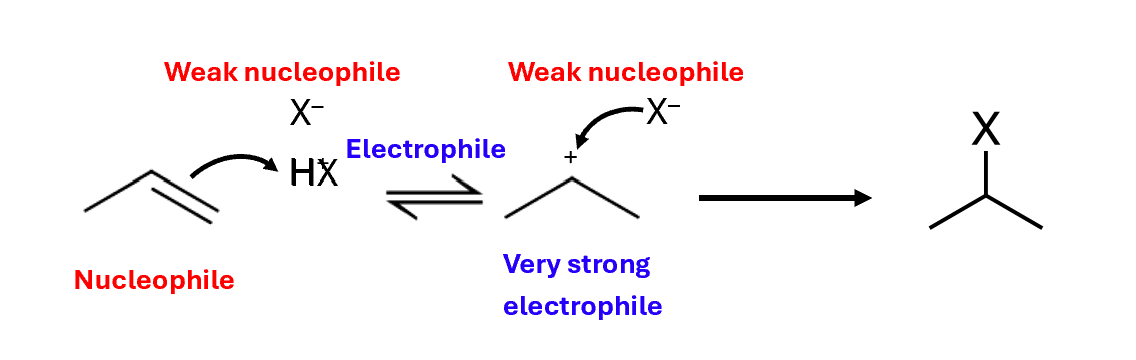
Electrophilic addition of HX to alkenes
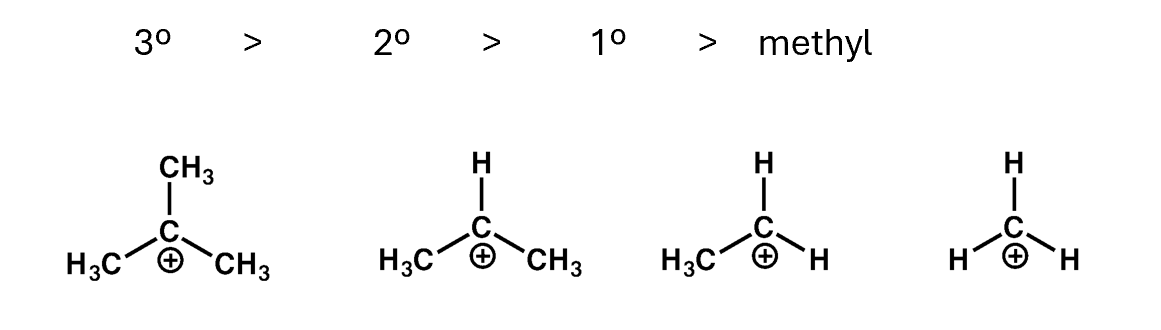
Carbocation stability
Alkyl groups help to stabilise carbocations
Therefore, tertiary carbocations are the most stable and the methyl carbocation is least stable
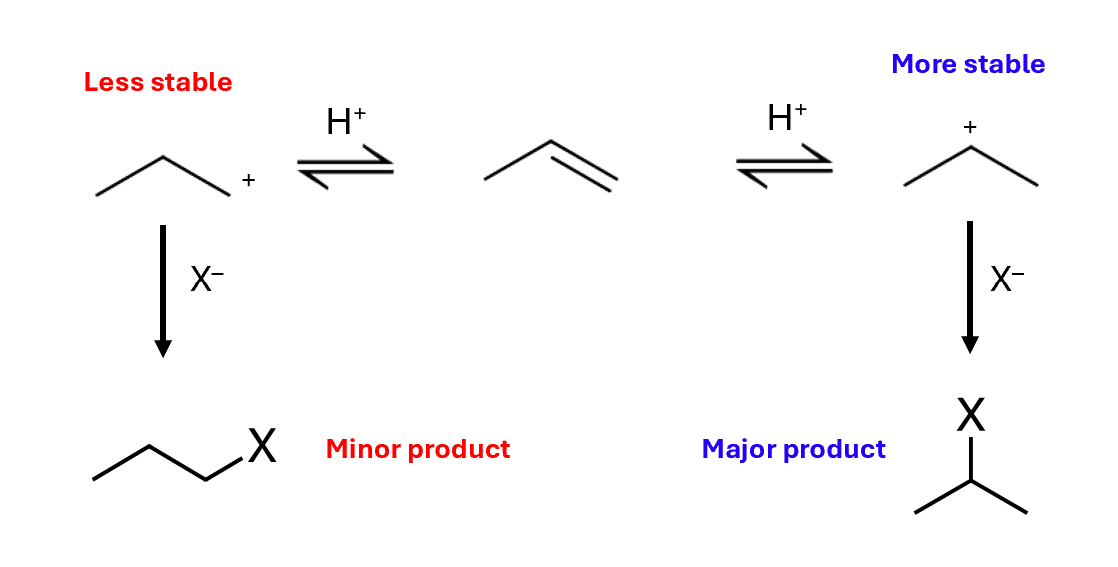
Forming major and minor products
More stable secondary carbocation is more likely to form
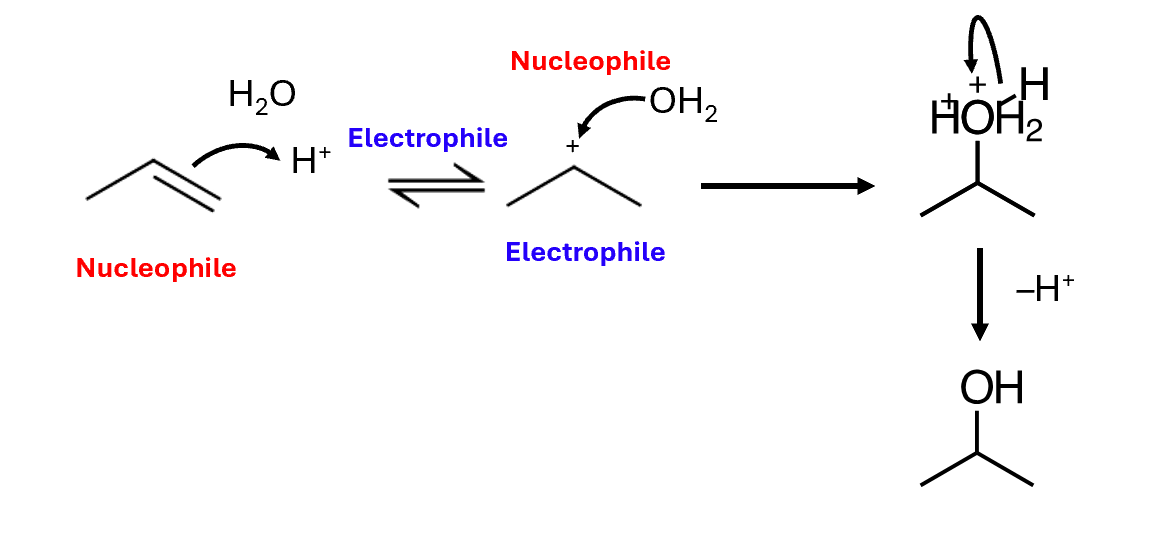
Hydration of alkenes
Electrophilic addition of X2 to alkenes