ap bio u3
1/99
Earn XP
Description and Tags
definitely contains more info than what's necessary for the ap test
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
why are organic molecules called "fuel molecules?"
they hv weak cov bonds, so they require lots of energy to stay together -> are rich sources of chemical energy
metabolic pathway
series of chemical reactions in which molecules are broken down or built up
what is ATP
a molecule that contains energy in its chemical bonds
where's ATP come from
as large molecules (w lots of CE) -> smaller molecules (less CE), chemical energy is released & packaged by cells into ATP
fuel molecules (lots of PE) -> ATP -> drive cellular processes
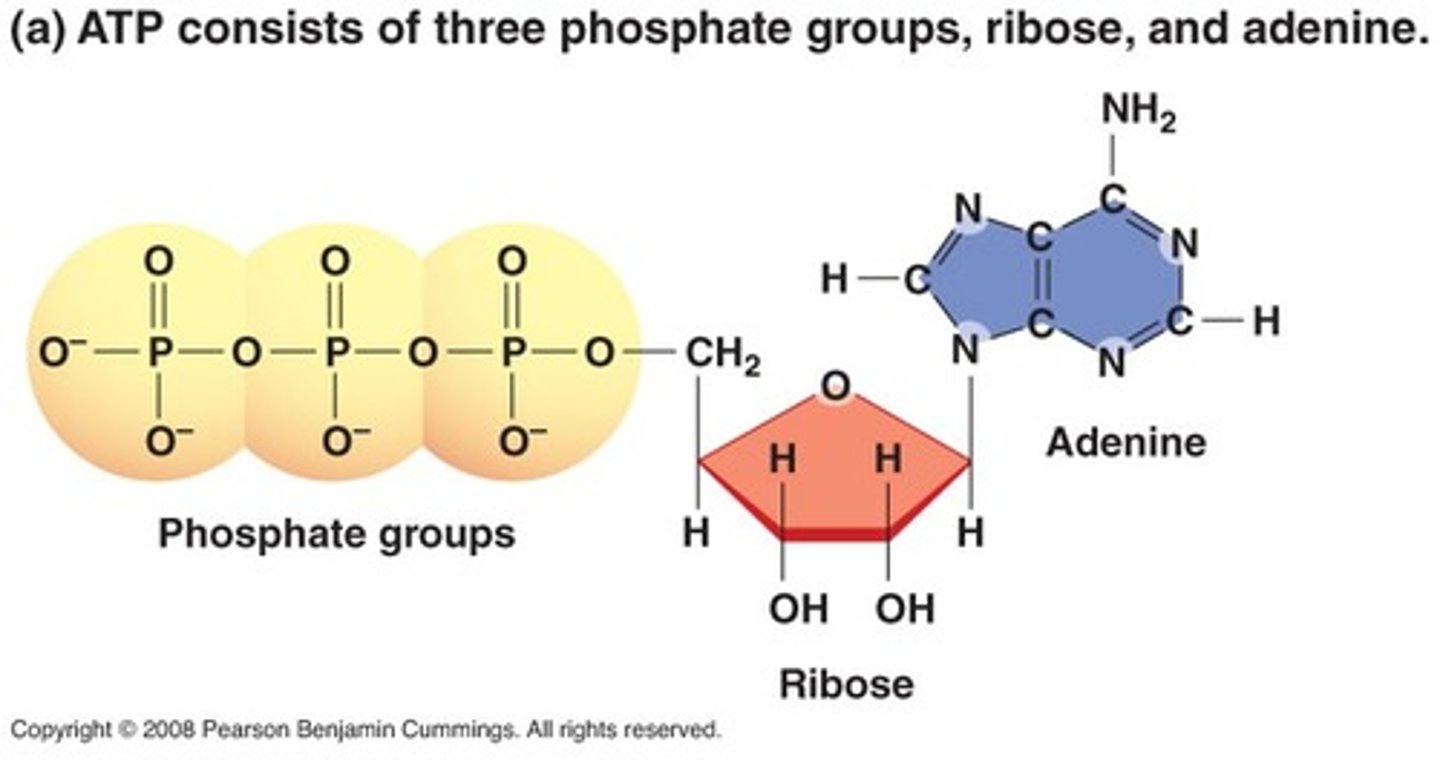
chemical structure of ATP
triphosphate + adenine + 5 carbon sugar (ribose)
wheres the accessible chem energy of ATP held? and why?
btwn bonds connecting phosphate groups
the bonds have high PE because they repel (neg charges) so when new bonds r formed, the excess is released
1st law of thermodynamics
energy cannot be created or destroyed, only transformed
2nd law of thermodynamics
every energy transfer increases the entropy (or disorder) and becomes less efficient (less energy available for use)
whats adding energy do to the order of a system
adding energy -> increase order of system
how do cells/organisms remain so organized despite all the energy transfers/transformations?
they're made up of many parts, so the heat can be released in the surrounding environment (aka entropy increases outside of the cell/organism)
gibbs free energy + equation
amt of available energy to do work
ΔG = products - reactants
endergonic/exergonic
ender: POS ΔG (REQUIRES ENERGY, NONSPONTANEOUS)
exer: NEG ΔG (RELEASES ENERGY, SPONTANEOUS)
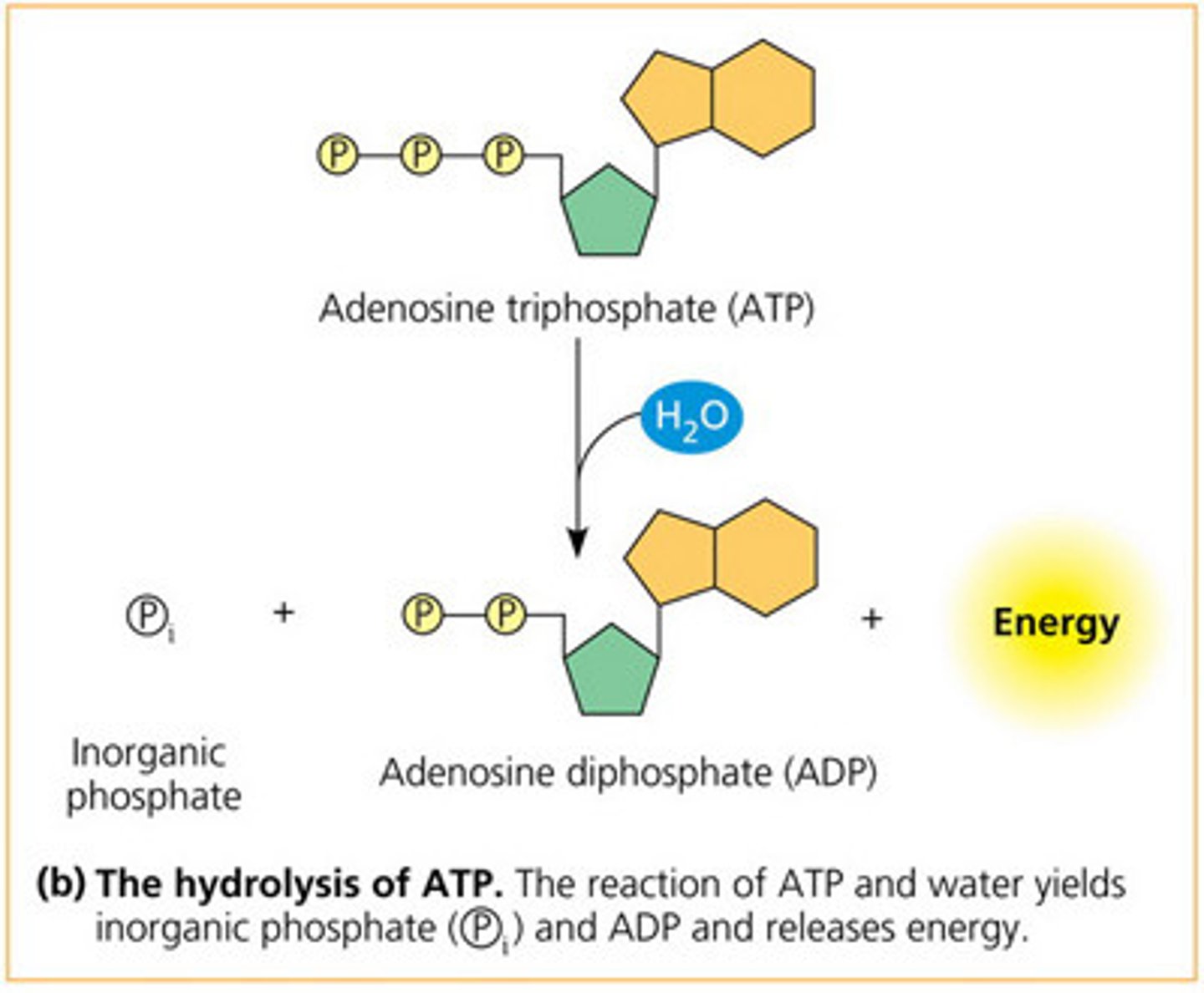
ATP hydrolysis
ATP -> ADP + Pi
released energy from forming more stable bonds fuels other processes
exergonic (neg ΔG)
energetic coupling
a spontaneous reaction drives a non spontaneous reaction
- sum of ΔG must be neg & they must occur tgt (sometimes shares a common molecule)
enzymes
protein catalysts in cells
transition state
a high-energy/unstable intermediate state where old bonds are breaking and new ones are being formed
how do enzymes work? (4 steps)
-> binds to reactants
-> forms complex which stabilizes transition state
-> reduces Ea
-> proceeds quicker
provides an alternative pathway that makes it more likely for reaction to proceed
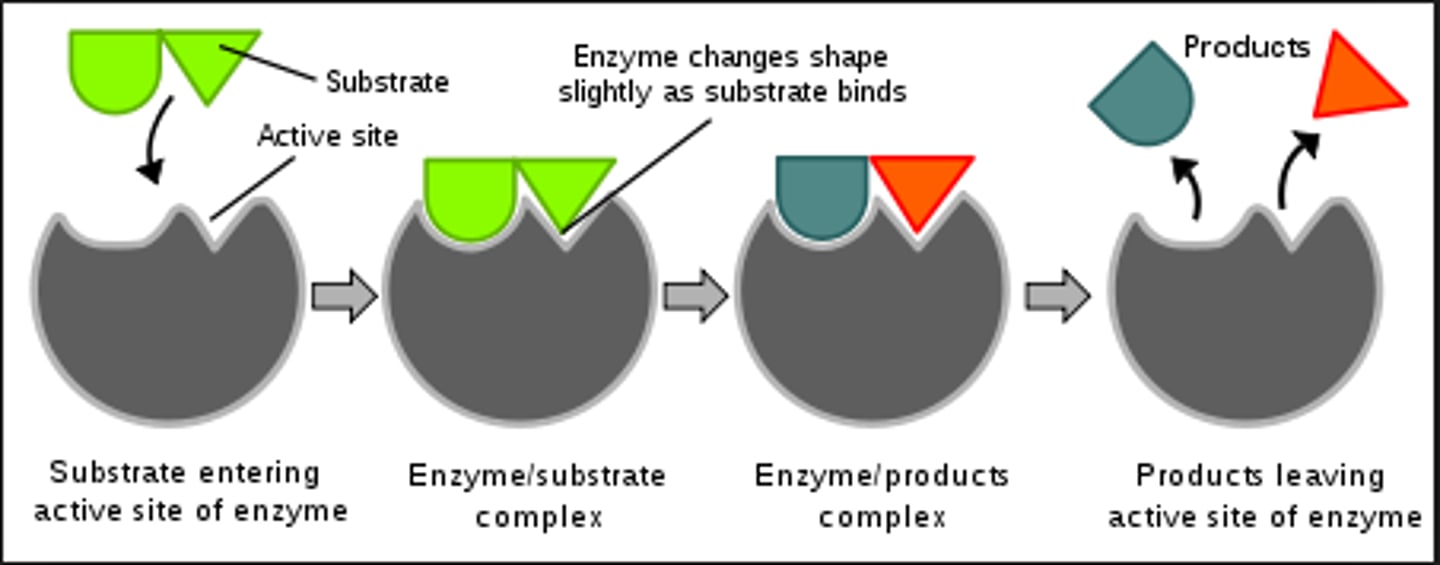
enzyme-substrate complex
E + S => ES => EP => E + P
S: substrate
E: enzyme
P: product
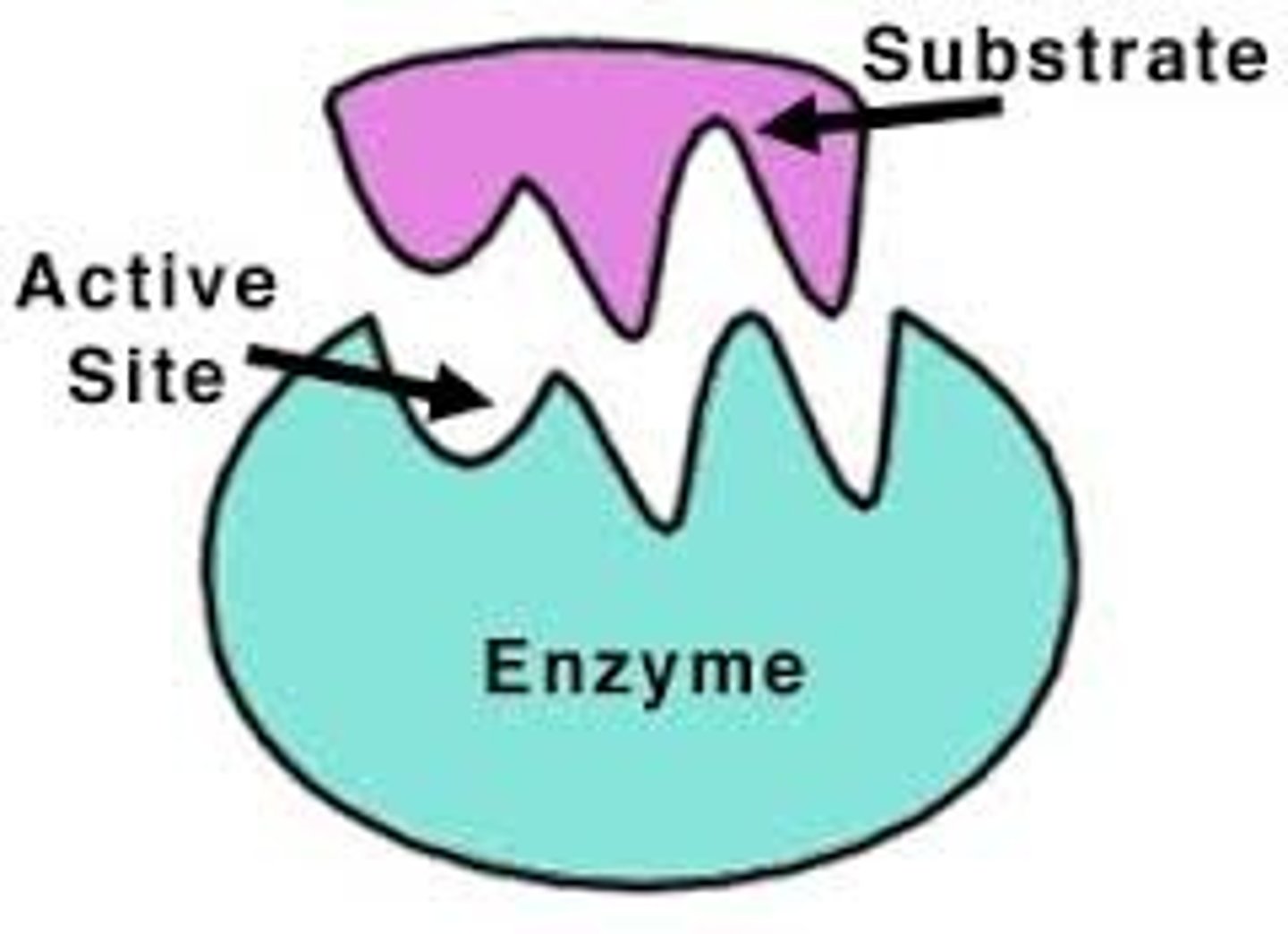
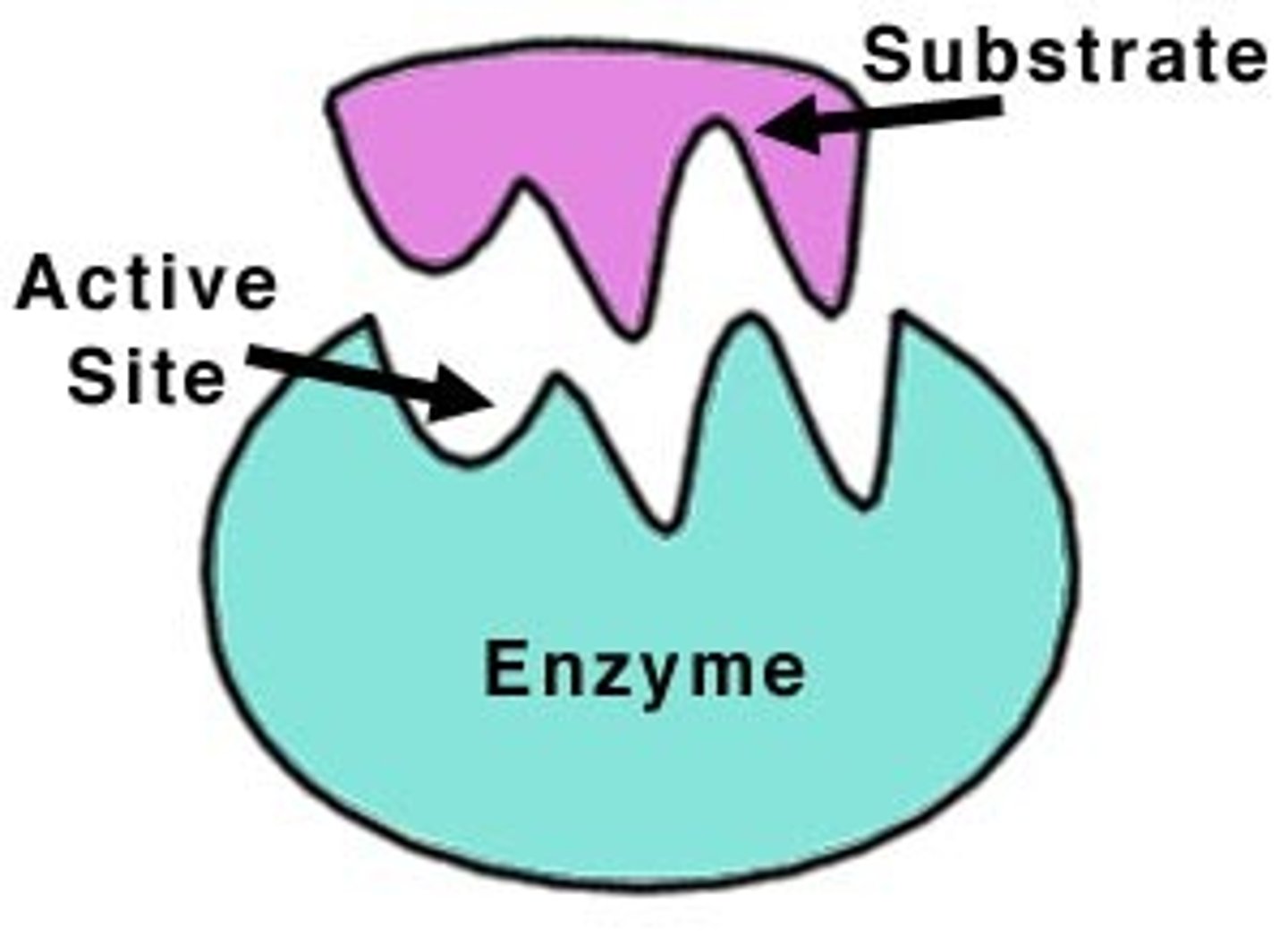
active site + types of bonds formed
the region of the enzyme where substrates are bonded to
- transient (v temporary) covalent bonds
- more commonly, noncov interactions (ionic, h-bonds, VDW)
what does the active site do?
substrate binds to it and it converts substrate to product
with more than one substrate, the active group aligns them & restricts their motion to increase probability of product creation
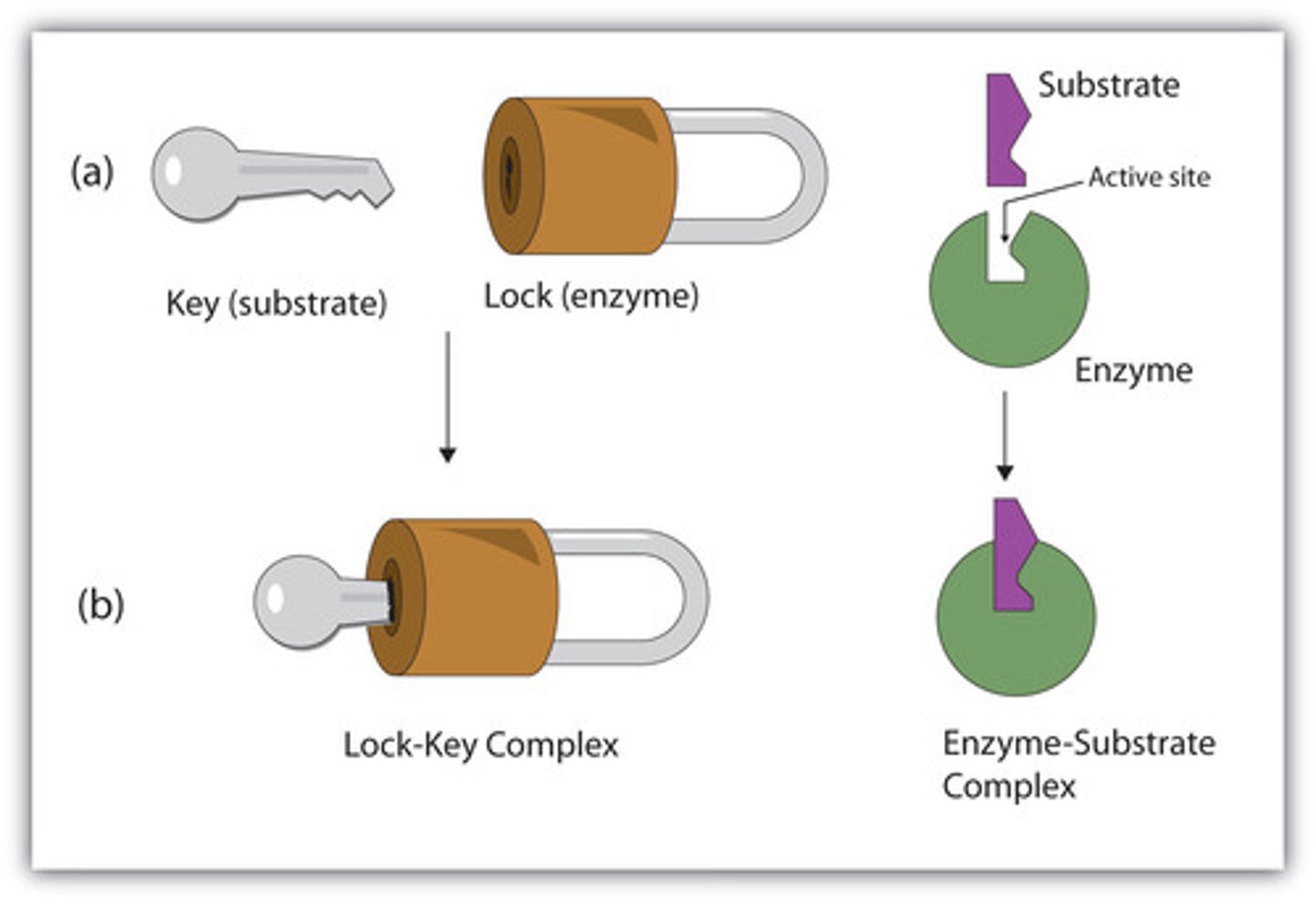
two models for binding of substrate?
lock & key + induced fit
lock & key model
enzymes are SPECIFIC for certain substrates
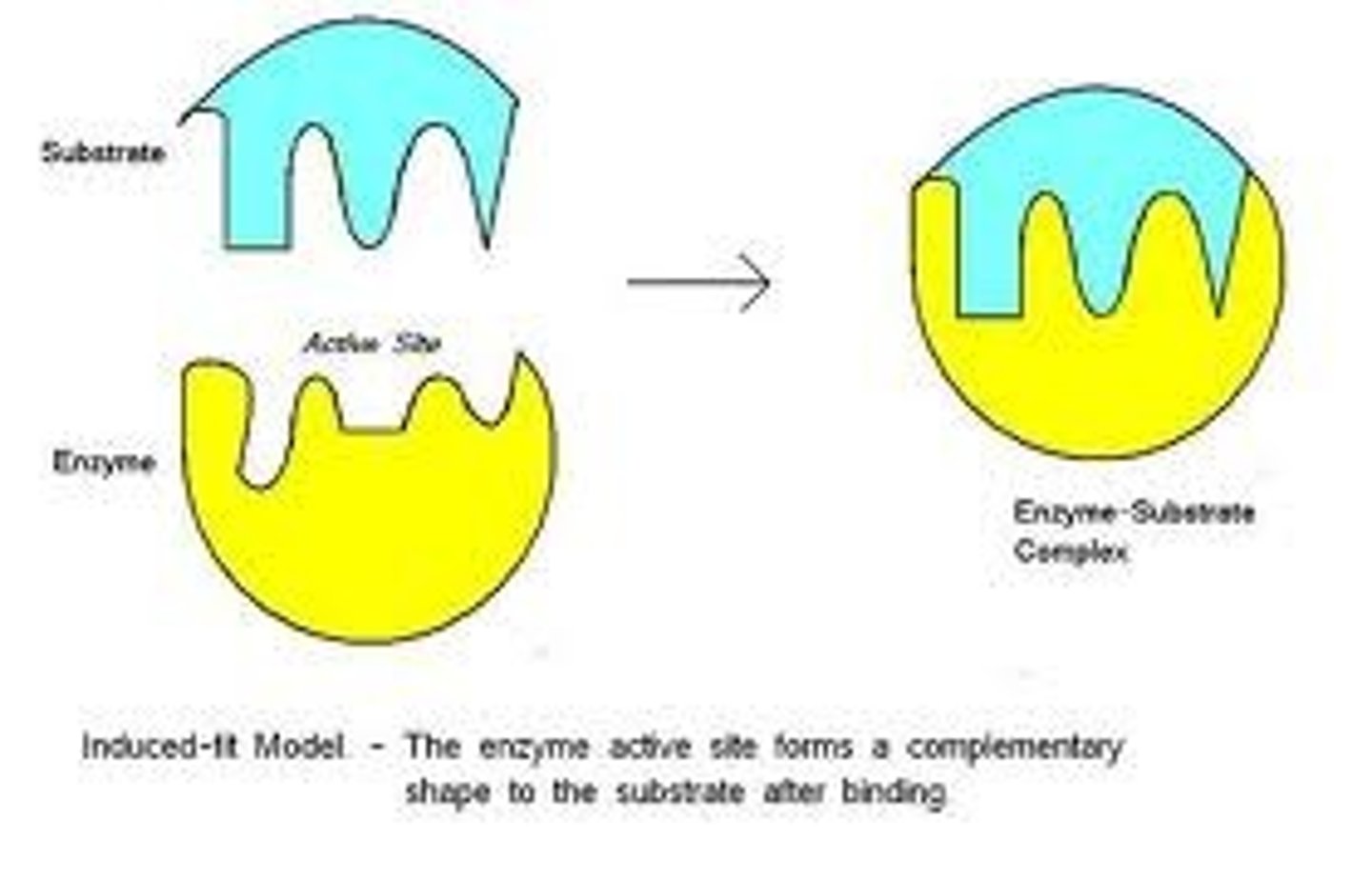
induced fit model
shape of active site is modified bcs substrate & active site are able to mold to each other
what happens to enzymes @ low & high temps
low temps: molecules move slower w less KE => reduces probability that reactants interact w enzymes
high temps: enzyme will unfold/denature & loses catalyzing abilities
chaperone proteins
proteins in cells that help protect slow-folding or denatured proteins until they attain proper structure (tho most happen super fast)
2 ways pH impacts enzymes & how
charges of amino acids:
=> lower pH (lots of H+): protons bind to functional groups (increase in + charges)
=> higher pH: the opposite (increase in - charges)
overall shape (protein folding):
changes amino acid charges and thus amino acid interactions as shape is formed
activators & inhibitors
compounds that increase activity of enzymes, decrease activity of enzymes
irreversible & reversible inhibitors
irreversible: forms cov. bonds & irreversibly inactivates enzymes
reversible: forms weak bonds & easily dissociates from enzymes
competitive inhibitors
binds to active site of enzyme (similar in structure w substrate)
SOLVED BY INCREASING SUBSTRATE CONCENTRATION
noncompetitive inhibitors
binds to different site (allosteric site) and therefore changes the shape & activity
CANNOT BE SOLVED BY INC. SUBSTRATE CONC.
allosteric enzymes
enzymes that are regulated by molecules that bind @ other sites (not active sites)
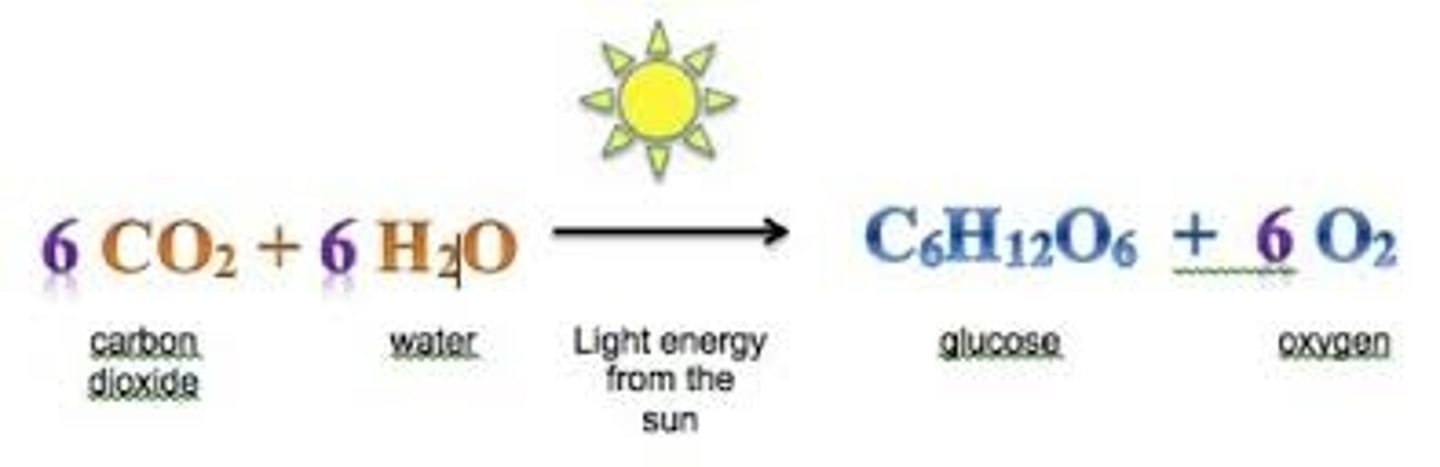
photosynthesis
using sunlight to turn H2O and CO2 into carbs (glucose)
NADPH/NADP+
a type of electron carrier in cells
oxidized: NADP+ (decrease in H cov. bonds)
reduced: NADPH (increase in H cov. bonds)
(NADP+) + (2e-) + (H+) => NADPH
*and vice versa
photosynthesis is a redox reaction
carbon partially gains e- (6CO2 => C6H12O6)
oxygen partially loses e- (6H2O => 6O2)
the two stages of photosynthesis
light reactions and calvin cycle
light reactions (light dependent)
1. chlorophyll absorbs sunlight energy
2. electrons move along photosynthetic electron transport chain
3. NADPH & ATP is produced
inputs: ADP, water, NADP+
outputs: ATP, O2 (g), NADPH
calvin cycle (light "independent")
CO2 -> carbs
*doesn't DIRECTLY use sunlight, but still needs it for the ATP & NADPH
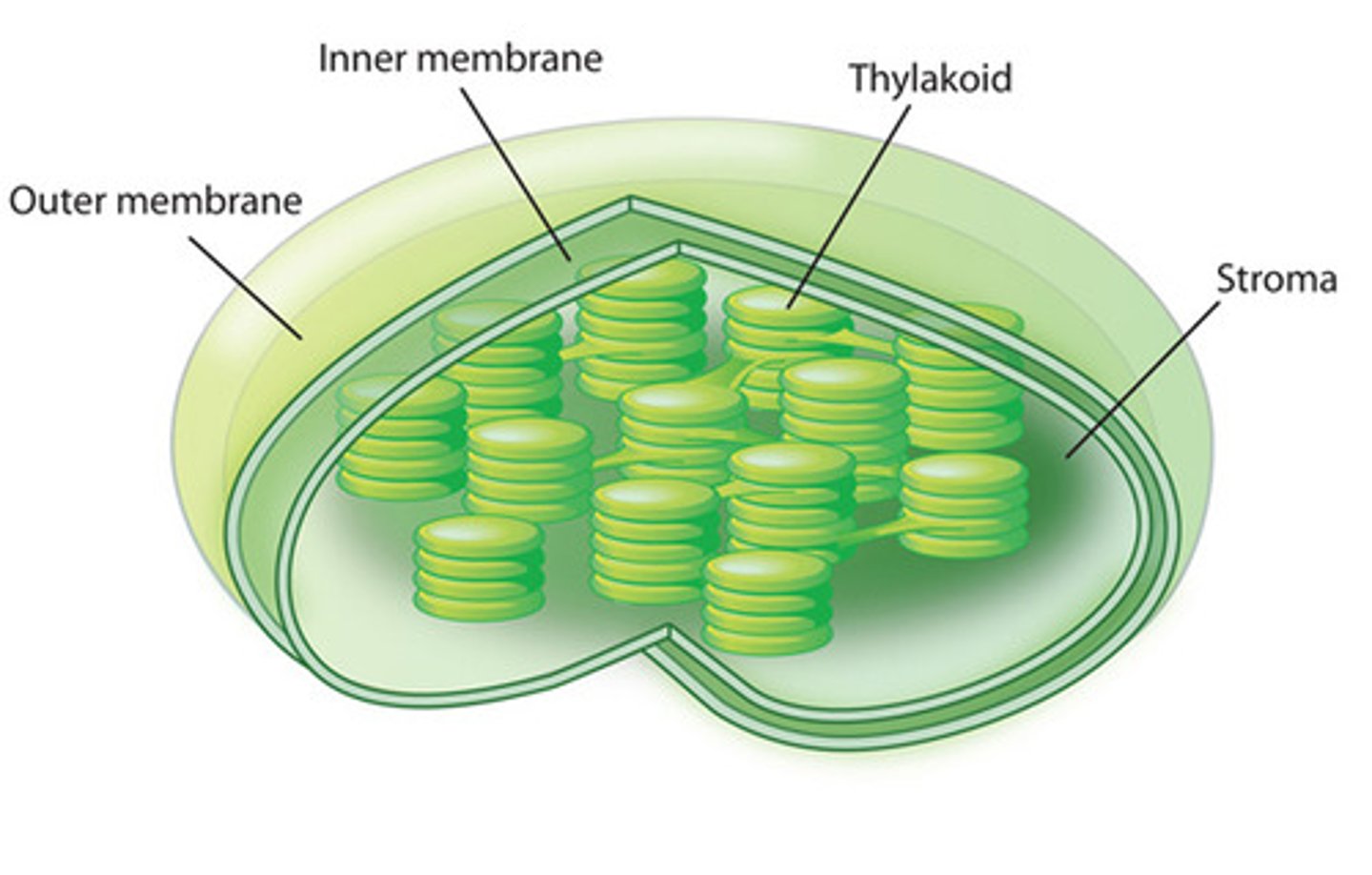
where does each part take place?
light reactions: thylakoids (stacks = grana)
calvin cycle: stroma
pigments
absorbs some wavelengths of visible light
chlorophyll
the major photosynthetic pigment (entry point for light)
*has two types: chlorophyll a &b
chlorophyll within an intact chloroplast in a lab vs in a cell
in lab: absorbed light energy is released (and chlorophyll then returns to ground state)
in cell: energy is transferred to an adjacent chlorophyll molecule (very little energy is lost as heat)
antenna chlorophyll
the chain of chlorophyll molecules where energy is transferred
reaction center
includes a pair of chlorophyll a molecules that accept & lose e'
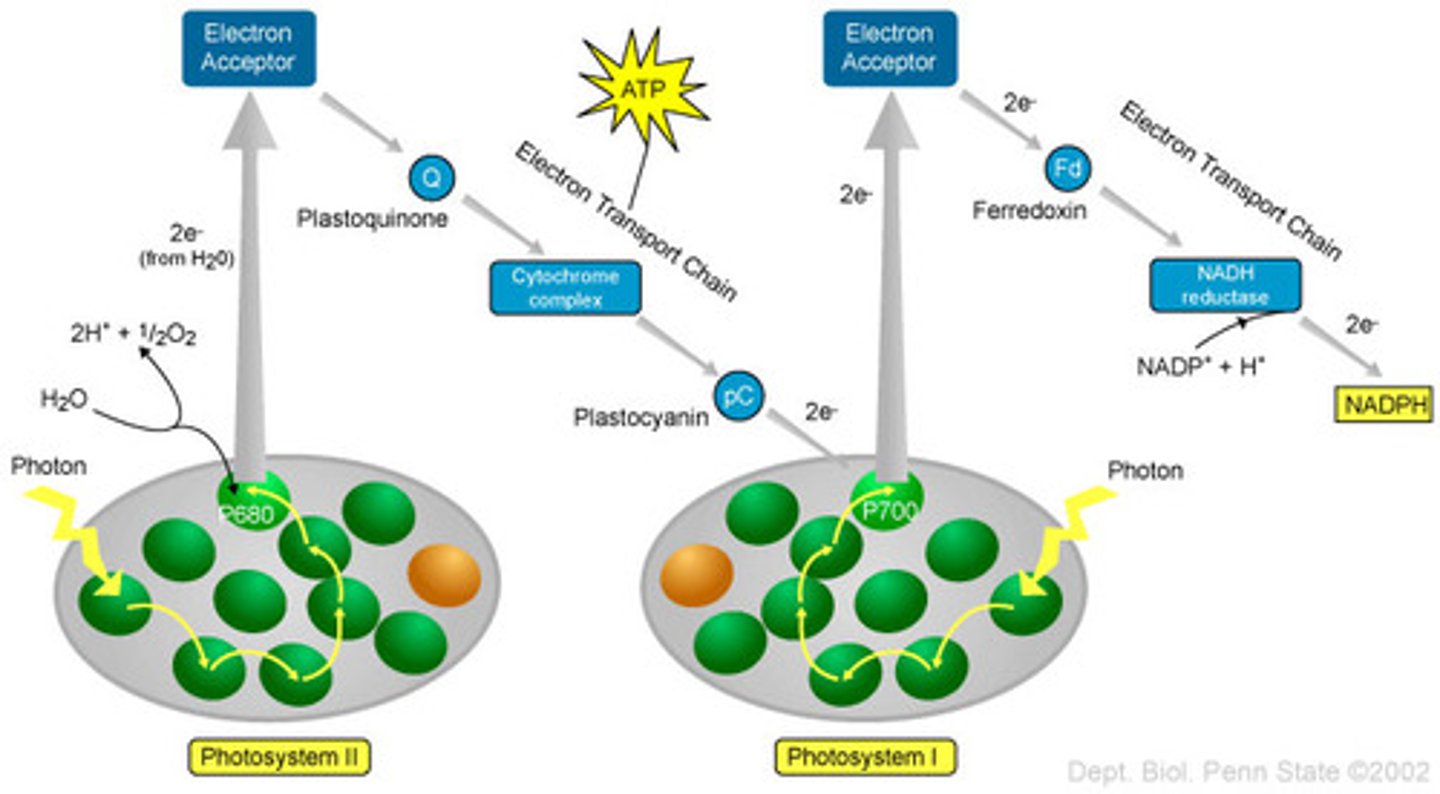
When excited, the reaction center transfers an electron to an adjacent molecule that acts as an electron acceptor. When the transfer takes place, the reaction center is oxidized and the adjacent electron-acceptor molecule is reduced. Once the reaction center has lost an electron, it can no longer absorb light or contribute additional electrons. Thus, another electron must be delivered to take its place. These electrons come from water.
photosystems
protein-pigment complexes that absorb light energy & drive electron transport
flows from photosystem II -> photosystem I
photosystem II
energy captured by II allows electrons to be pulled from water
photosystem I
energy captured by I allows electrons to be transferred to NADP+ to form NADPH
three sections of the Z scheme (photosynthetic electron transport chain)
light energy is first absorbed to pull electrons from H+
what r the ways protons accumulate inside thylakoid
from oxidation of water
as e- move along chain, protons are pumped from stroma -> thylakoid
proton gradient/electrochemical gradient
SOURCE OF POTENTIAL ENERGY (STORES IT) bcs thylakoid wants to go to stroma (except membranes r only selectively permeable)
chemical vs elec gradients
chem gradient: diff in concentrations btwn sides of membrane
elec gradient: diff in charges btwn 2 sides of membrane
ATP synthase (general & specific idea)
ATP synthase uses light energy to synthesize ATP
enzyme that uses KE from proton movement down electrochem gradient (from thylakoid => stroma) to synthesize ATP
ADP + Pi => ATP
photophosphorlyation
(light energy powered) process by which phosphate is added to a molecule (ADP + Pi)
entire process (sunlight-> synthesizing atp)
sunlight energy is absorbed by a chlorophyll (a/b) and the energy is passed btwn chlorophylls inside PS II.
electron eventually reaches the reaction center (contains special pair of c.a). the electron is then transferred to primary electron acceptor
so, H2O is added to final chlorophyll RC to reduce it.
meanwhile, electron travels down photosynthetic ETC (releases H+ as it goes) till it reaches PS I where electron loses energy (light energy then gives it energy again)
also in PS II, water also releases H+ and O2 (along with the e-)
the H+ from the past two steps travel down concentration gradient thru ATP synthase to combine with ADP to form ATP
the e- in PSI travels down its own ETC and electron is released where it binds with NADP+ to form NADPH
the calvin cycle (occurs in light y/n?)
series of enzymatic reactions that synthesize carbs from CO2
(doesn't directly use sunlight, but needs NADPH & ATP => only occurs in the light)
1. carbon fixation + catalyzed by what?
CO2 in air is converted to organic molecule in a cell
CO2 + RuBP (5-carbon molecule) = 6-carbon molecule
=> breaks into two 3-carbon molecules
*catalyzed by rubisco
2. reduction
3-carbon molecules => carbs (triose phosphates/G3P)
*requires ATP & NADPH
triose phosphates/G3P
true products of calvin cycle & principal form of carbs exported from chloroplast during photosynthesis
(later converted into glucose)
*CYCLE MUST REPEAT 6 TIMES BCS EACH CYLE ONLY ONE G3P IS RELEASED
3. regeneration
G3P + 3 ATP => regenerate 5-carbon sugar (RuBP)
*requires ATP
what happens to the excess carbs (G3P) that's produced + two benefits
converted to starch (storage form of carbs) through dehydration synthesis
=> stored in granules that aren't water soluble (doesn't lead to osmosis)
=> provides photosynthetic cells w source of carbs during night
substrate
reactant of an enzyme-catalyzed reaction
THING THAT ATTACHES TO ENZYME
photorespiration
rubisco adds O2 to 5-carbon molecule => CO2
*ATP is consumed = net energy drain
*oxidizes and loses carbon atoms
why does photorespiration still happen then?
due to the similar in size & structure of CO2 and O2, rubisco struggles to distinguish between them
to increase its selectivity for CO2, its reaction speed is slower
=> rubisco must be produced in large quantities
however the abundance of O2 in atmosphere means photorespiration still causes loss of carbon
what happens if light reactions proceed quicker than calvin cycle
NADP+/ADP will be in short supply, electron transport chain backs up (excess e- and energy)
=> creates reactive oxygen species (can cause substantial damage to cell by oxidizing macromolecules)
*most likely under high light intensity (such as midday)
*cold temperatures reduce rate of NADPH/ATP production (and increases ROS production)
how do photosynthetic organisms avoid stresses that arise from calvin cycle not being able to keep up??!
chemicals that detoxify ROS: exists in high conc in chloroplasts
prevent ROS from forming at all:
ex. xanthophylls (reduces excess light energy by converting it to heat)
cellular respiration
metabolic pathway that converts energy in carbs/fuel molecules => chemical energy in ATP
SYNTHESIZE ATP FOR USE BY CELL
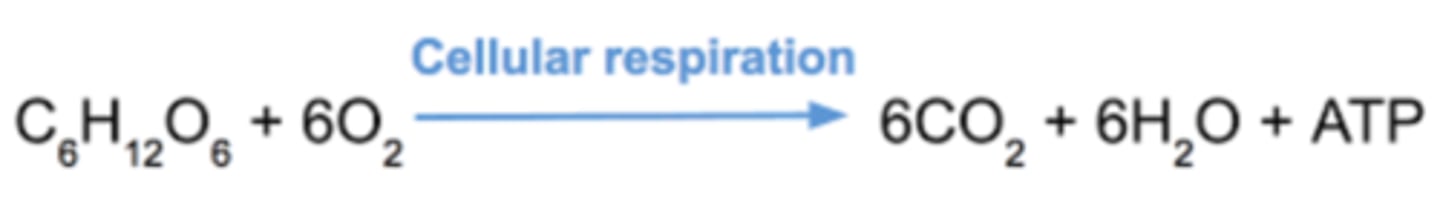
cellular respiration overall reaction
glucose + oxygen -> co2 + water + energy
what's oxidized and what's reduced in cellular respiration?
oxidized: C6H12O6 => CO2
(carbon goes from equally shared to all towards EN oxygen)
reduced: O2 => H2O
(equal sharing => gained electrons go towards EN oxygen)
two ways of ATP production
substrate-level phosphorylation
1. hydrolysis of molecule to yield phosphate group
2. addition of P group to ADP (from enzyme substrate)
oxidative phosphorylation
1. energy transferred to electron carriers (NAD+ & FAD)
2. they each gain 2 e- from the oxidation of fuel molecules
*driven by pumping of protons (electrochem gradient)
*oxygen is final electron acceptor & produces water
whys cellular respiration necessary?
1. tho photosynthetic organisms make their own carbs, they need ATP bcs its cell's usable & ready form of energy
(ATP produced during photosynthesis is for calvin cycle)
2. used at night when theres no light energy to make ATP
photosynthetic vs respiratory ETC (energy & location)
P: harnesses sunlight energy
R: harnesses energy from fuel molecules
P: in thylakoid membrane
R: located in inner membrane of mitochondria
4 stages of cellular respiration
glycolysis, pyruvate oxidation, krebs cycle, oxidative phosphorylation
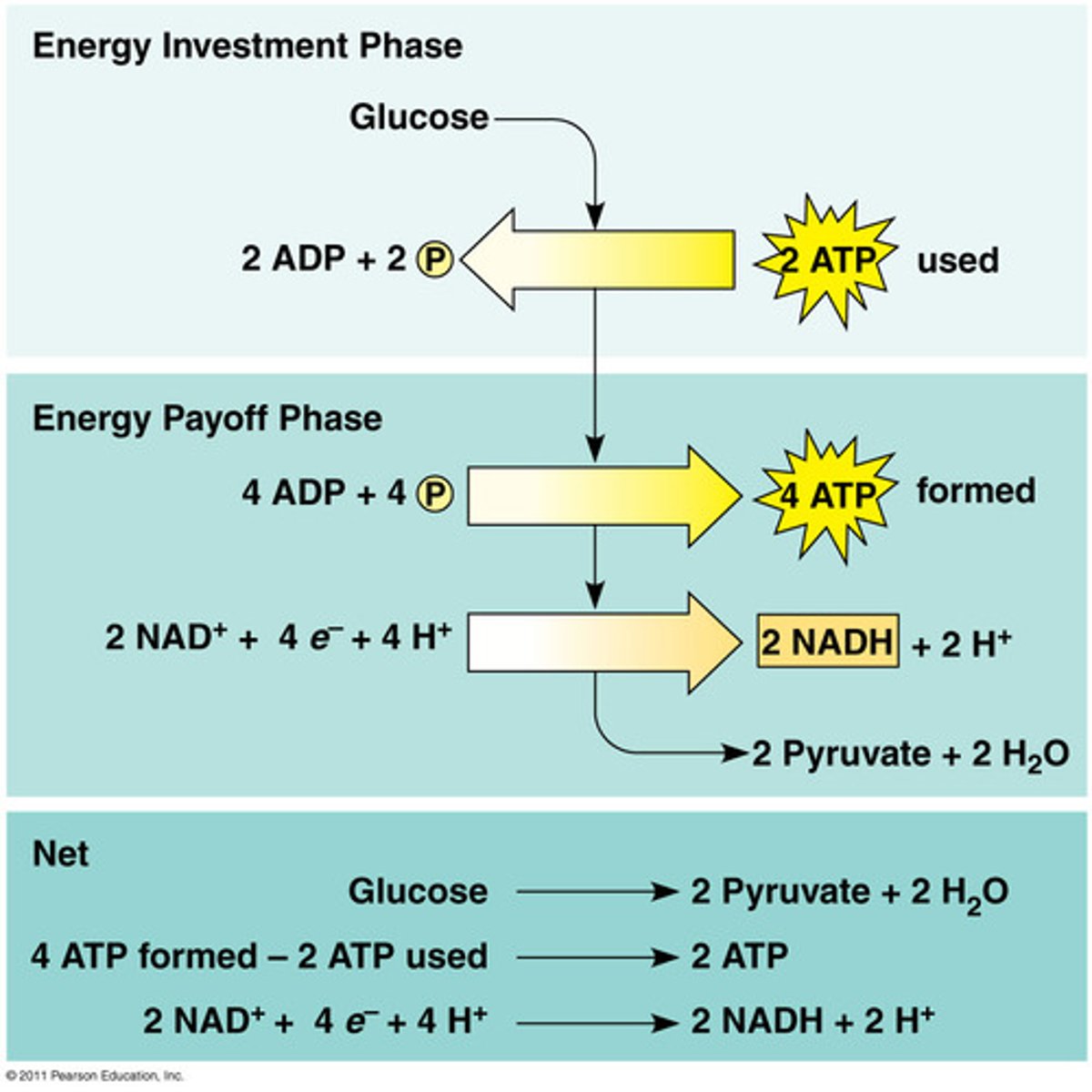
1. glycolysis (action, products, location)
ACTION: glucose is broken down to pyruvate
PRODUCTS: 2 ATP (4-2), 2 NADH, 2 pyruvate
LOCATION: in cytoplasm
glycolysis initial reactants & final products
initial: 6-carbon molecule of glucose
products: two 3-carbon molecules of pyruvate
NET TOTAL: 2 ATP & 2 NADH
*directly thru SLP
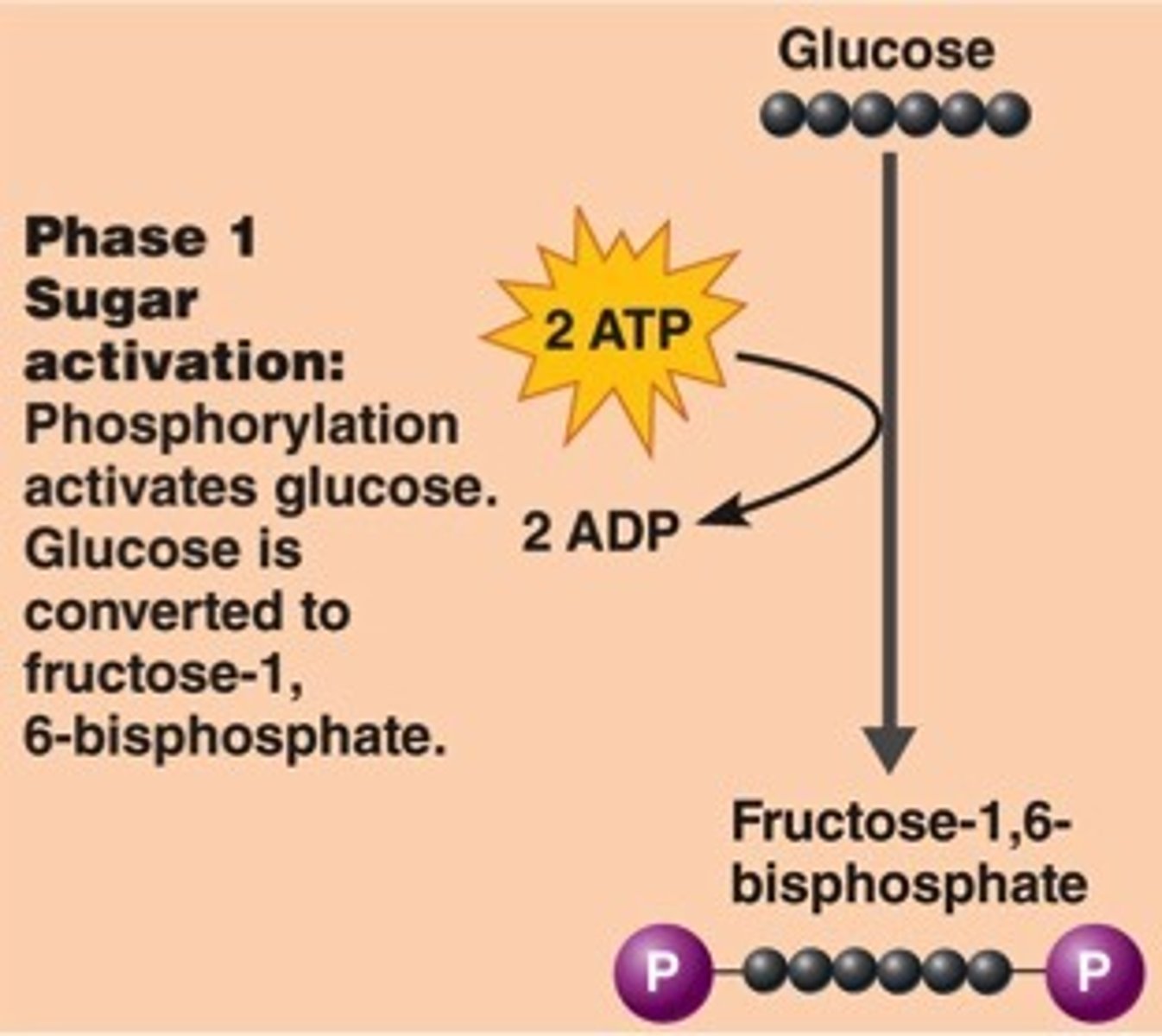
phase 1 of glycolysis + why?
preparatory phase: adds 2 phosphate groups to glucose
1. confines product of reaction to INSIDE the cell
2. neg charged P groups repel & destabilized molecule so it can be broken apart (in stage 2)
REQUIRES ENERGY (ENDERGONIC)
=> 2 ATP is used per molecule of glucose
phase 2 of glycolysis
cleavage phase: 6C molecule => two 3C molecules
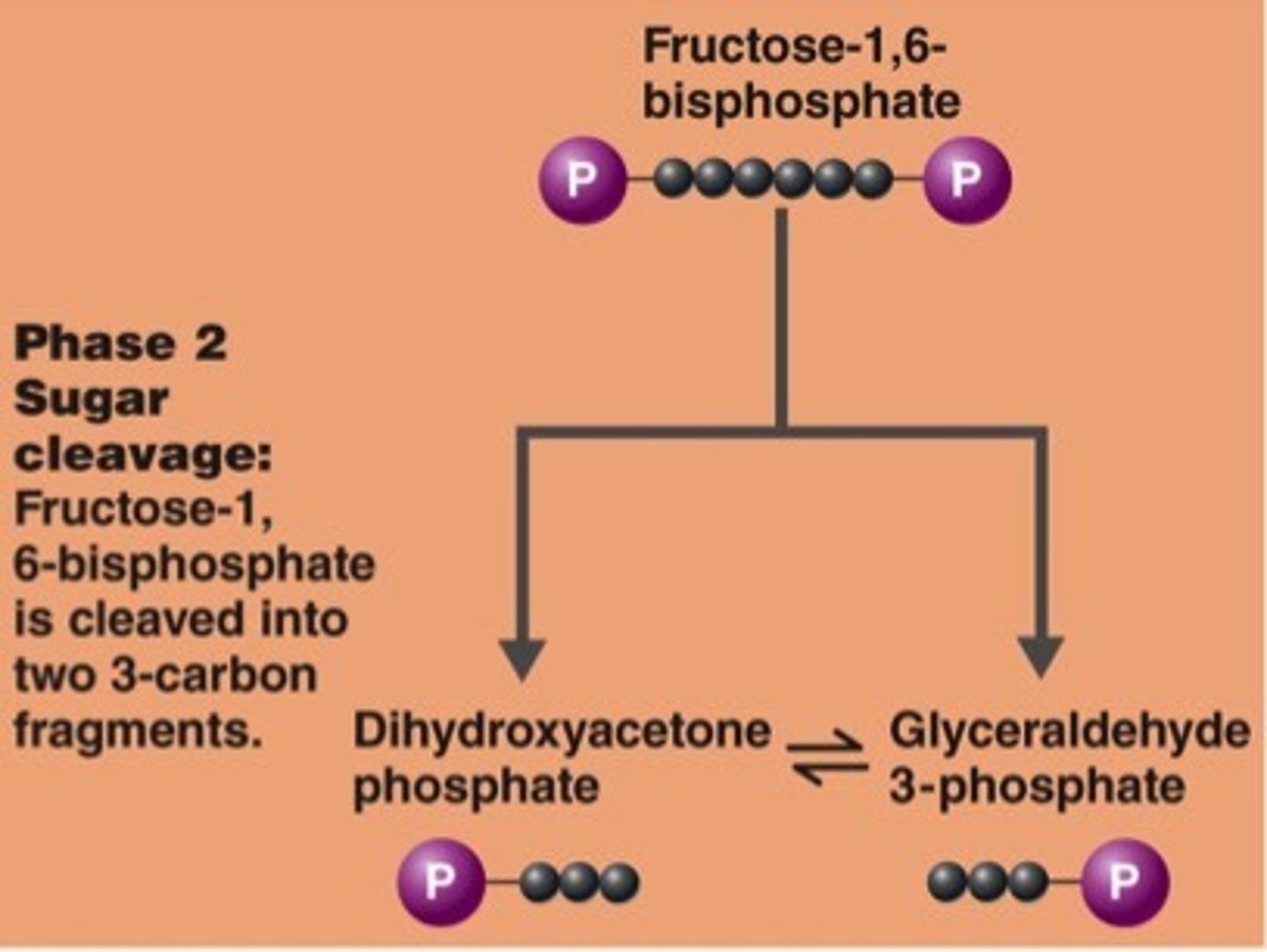
phase 3 of glycolysis
payoff phase: 4 ATP, 2 NADH, and two molecules of pyruvate are produced
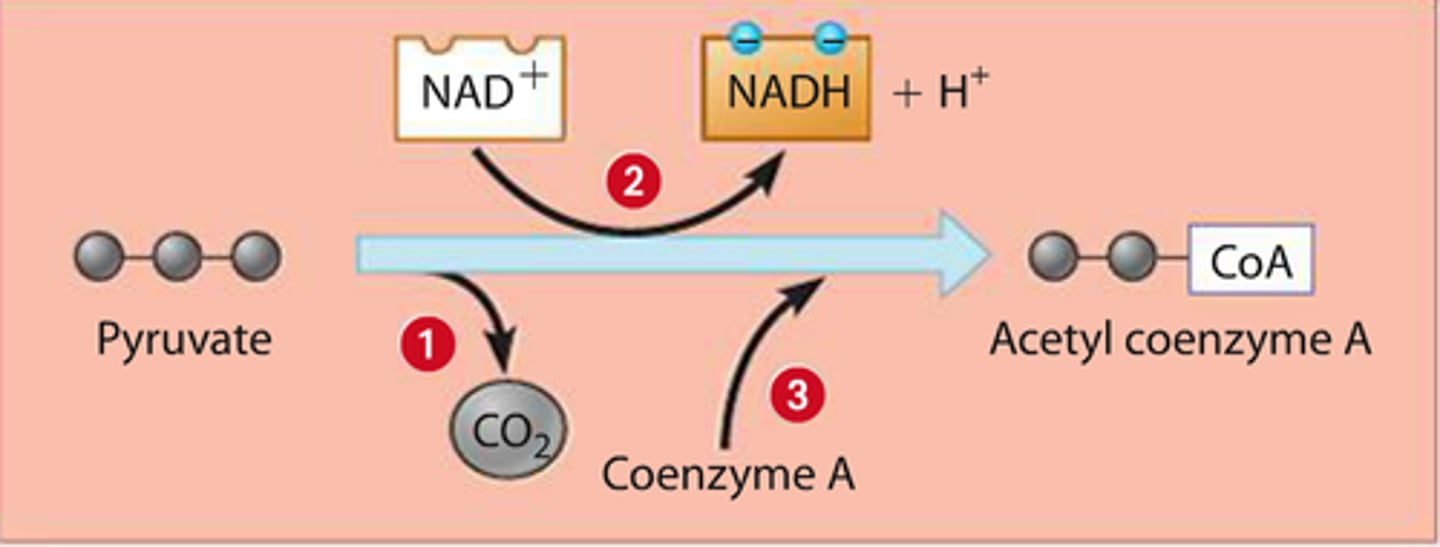
2. pyruvate oxidation (action, products, location)
ACTION: pyruvate is oxidized to acetyl-coenzyme A (acetyl-CoA)
PRODUCTS: 2 NADH, 2 acetyl-CoA, 2 CO2
*co2 = waste, NADH forms from NAD+ and e- lost by pyruvate
LOCATION: mitochondria
acetyl group (COCH3)
transferred to coenzyme A (CoA) which carries acetyl group to next step
(contains large amt of PE)
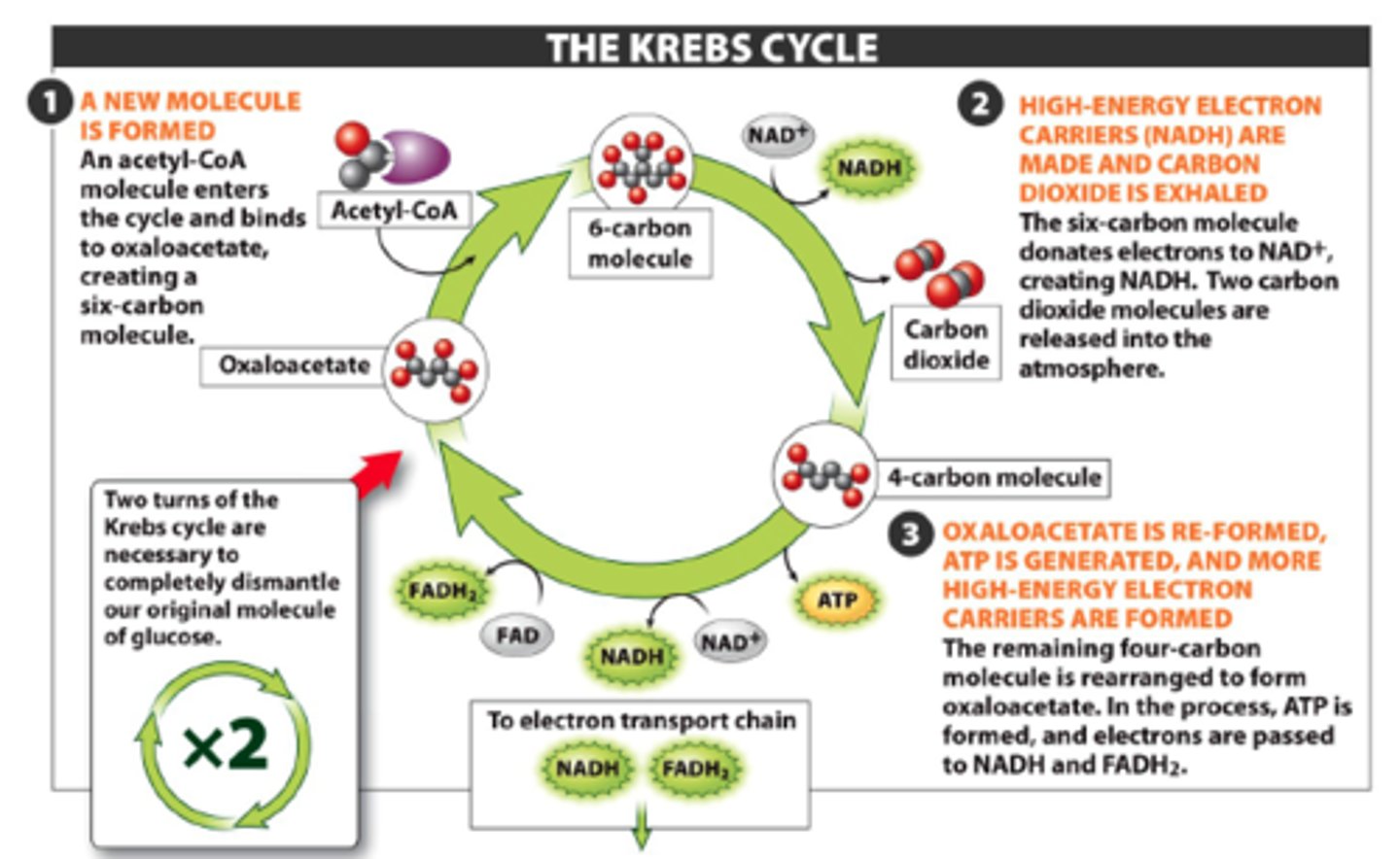
3. krebs cycle/citric acid cycle (action, products, location)
ACTION: acetyl group oxidized to CO2, free energy is transferred to ATP (SLP)
PRODUCTS: 2 ATP, 6 NADH, 2 FADH2, 4CO2
LOCATION: mitochondrial matrix
*PRODUCES MUCH MORE ENERGY THAN IN STAGES 1 & 2
first part
2C acetyl-CoA + 4C oxaloacetate = 6C citric acid
second part
four redox reactions produce three NADH & one FADH2
these carriers donate e- to ETC
third part
citric acid is oxidized in series of reactions where the last reaction regenerates 4C oxaloacetate (joins 2C and cycle continues)
4. oxidative phosphorylation (action, products, location)
ACTION: FADH2 & NADH donate e- to respiratory ETC
PRODUCTS: ATP, H2O, NAD+ & FAD
LOCATION: inner mitochondrial membrane
*PRODUCES THE MOST ATP
respiratory ETC is made up of
four large protein complexes (complexes I to IV) embedded in inner mitochondrial membrane
donated electrons enter where? what happens to them?
donated by NADH: complex I
donated by FADH2: complex II
(then transported thru III and then IV) => OXIDIZED
redox couples
within each protein complex, e- is passed from e- donor to acceptor (redox centers)
oxidized & reduced form = redox couple
eg. oxygen accepting e- in the presence of H+ is reduced to H2O
proton gradient is caused by?
caused by oxidation of NADH & FADH2
how does proton gradient help (3 steps)
proton flow thru transmembrane protein makes it possible for ATP synthase to create ATP
1. proton gradient makes channel rotate
2. PE is converted into KE (mech rotational energy)
3. enzyme changes shape which allows it to ADP -> Pi
chemiosmosis
protons moving across membrane + synthesis of ATP
how much ATP produced for each NADH & FADH2 (that donates e- to the chain)?? total ATP produced?
NADH: 2.5 molecules
FADH2: 1.5 molecules
OVERALL: 38 molecules of ATP from all 4 stages of CR
uncoupling agents
protons that spanning inner membranes that allow protons to bypass ATP synthase channel
=> decrease proton gradient & levels of ATP
=> energy of proton gradient is dissipated as heat
*found naturally for heat generation, also are poisons
what types of reactions lead to production of ATP & reduced e- carriers
exergonic reactions (no outside energy necessary, decrease in free energy)
how is excess glucose stored?
glycogen in animals, starch in plants
other molecules that can produce ATP are?
oxidizing fatty acids can provide a large amt of ATP, BUT can't be used by all tissues of body (ex. RBC's and the brain)
proteins broken down to amino acids enter at various points in stages 1-3, but are a last resort energy source bcs they hv other roles (structural, etc.)
what happens after glycolysis in anaerobic respiration?
concentration of pyruvate builds up in cytoplasm
how can the pyruvate be broken down then/how can NAD+ be regenerated?
fermentation: metabolic pathways that extracts energy from fuel molecules w/ out O2 or ETC
whens fermentation used
organisms w out oxygen, for yeast/others that favor fermentation, and also when oxygen cant be delivered quickly enough (eg. exercise)
lactic acid fermentation
e- from NADH are transferred to pyruvate
Glucose + 2 ADP + 2Pi => 2 Lactic acid + 2 ATP + 2H2O
bacteria & animals (yeast)
ethanol fermentation
pyruvate releases CO2 => acetaldehyde
acetaldehyde + e- (from NADH) => ethanol & CO2
Glucose => Ethanol + 2CO2 + ATP
plants and fungi (muscles)
ATP yield of fermentation
2 molecules of ATP
(end products aren't fully oxidized and still contain large amts of chemical energy => LOTS OF FUEL MOLECULES ARE NECESSARY TO POWER THE CELL)