Membrane structure
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
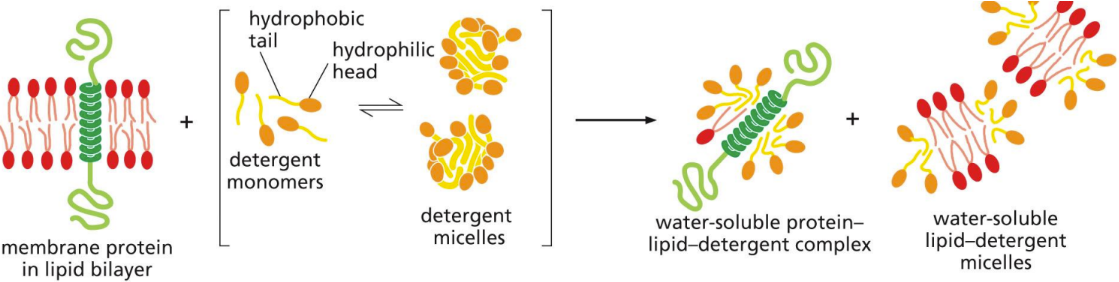
What is a detergent
small amphiphilic molecules of various structure
Hydrophobic ends of detergents bind to hydrophobic regions of membrane proteins and bring membrane proteins into solution
Good for studying membrane protein characteristics and activities
Pulls membrane proteins into solution (disrupting bilayer)
What is cholesterol
Serves to increase impermeability of the lipid bilayer and prevents hydrocarbon tails from crystallizing (is a sterol)
Found in the bilayer (lots in eukaryotes)
Supportive region to stiffen fatty acid tails
Oriented with its hydroxyl polar head close to polar heads of phospholipids
What is a ganglioside
complex glycolipids found mostly in nerve cells
- Tay shaches disease
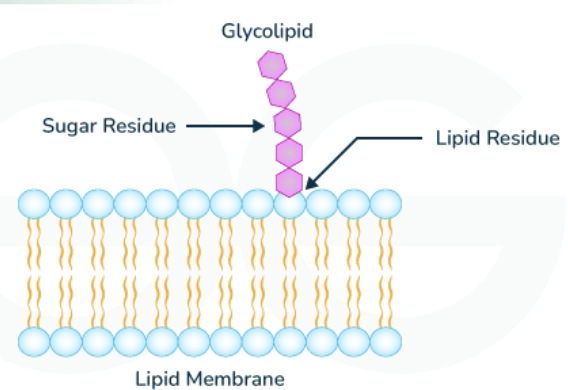
What is a glycolipid
sugar-containing lipid molecules found exclusively in noncytosolic side (exterior side of cell) of lipid bilayer
Sugars are added in lumen of Golgi apparatus (environment similar to extracellular)
Function = to help cell interact with its surroundings, protect from harsh surroundings, affect electrical field of membrane and ion concentration
What are membrane anchors (example)
example = Glycosylphosphatidylinositol anchor (GPI) = special membrane proteins that are actually extracellular made in the ER of the cell as single-pass initially
Transmembrane segment is cleaved and the GPI anchor is added
Anchor is only hold on this protein
Protein is delivered via transport vesicle to the cell membrane
GPI is the anchor
What is the lipid bilayer
Serves as relatively impermeable barrier to water soluble molecules
Thin film of lipid and protein molecules held together by non-covalent interactions
Not rigid because has 2 layers
Proteins embedded
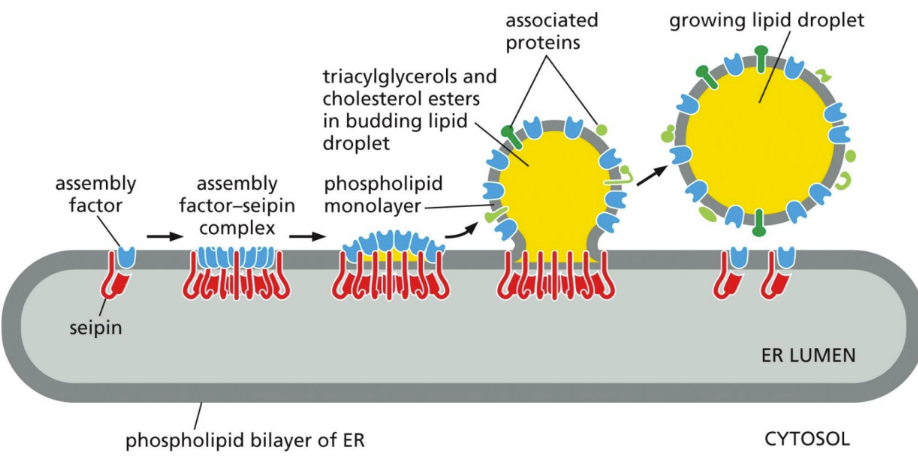
What is a lipid droplet
excess cellular lipids stored in organelles surrounded only by a phospholipid monolayer
Contain neutral lipids like triglycerides and cholesterol esters
Formed from ER membrane
Ex: adipocytes = just one big fat droplet
What is a phospholipid
most abundant membrane lipid
Has 2 hydrophobic hydrocarbon tails (fatty acids)
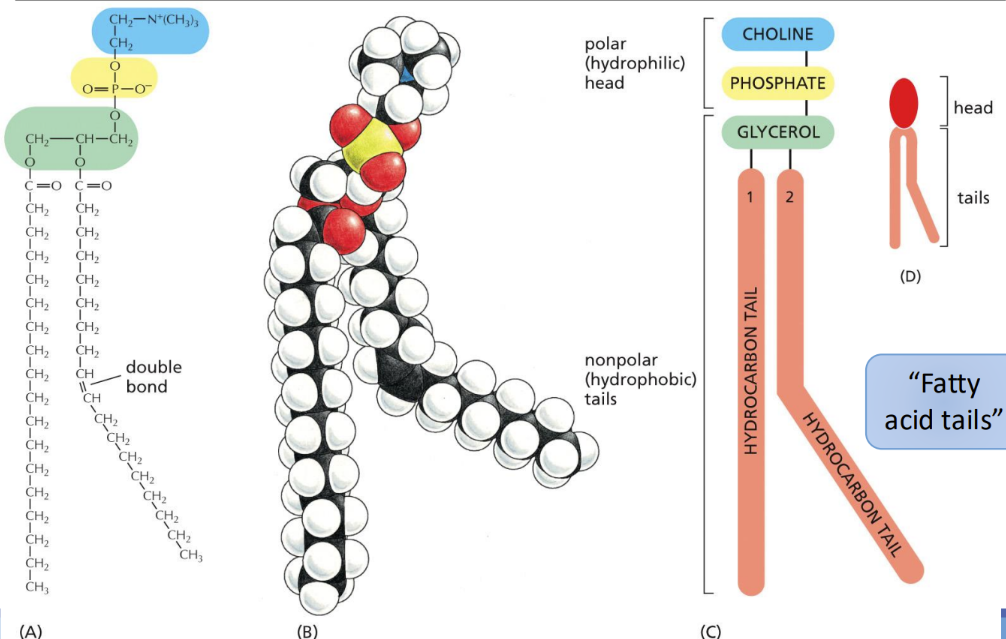
What is glycosylation
adding sugars to proteins
Most cell membrane proteins are glycosylated (contain sugars)
Sugars added in the ER and Golgi to proteins destined for some membrane
Sugars are always present on the noncytosolic side of membrane (extracellular side)
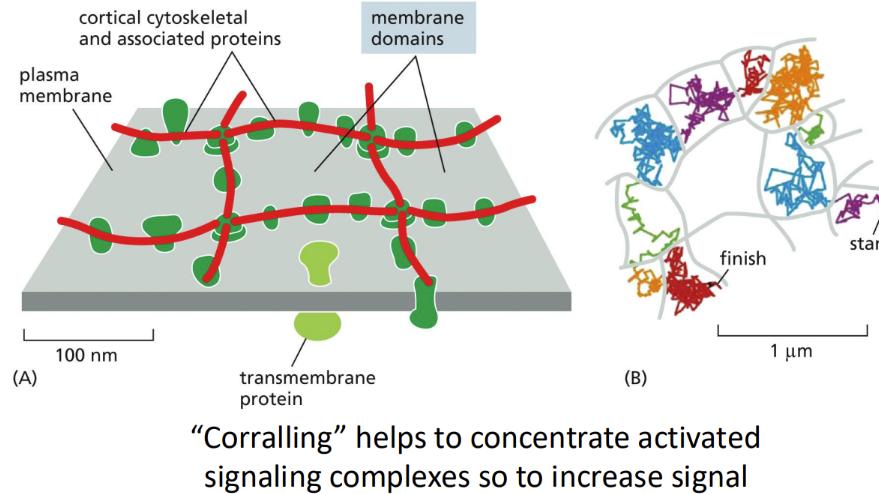
What is corralling
helps to concentrate activated signaling complexes so to increase signal
What are the common phospholipids
Most common phospholipids in mammalian cell membranes: 3-carbon glycerol backbone
Phosphatidylethanolamine = Found mainly in E. Coli & mitochondrion
Phosphatidylserine = found mainly in myelin & RBC
Phosphatidylcholine = found mainly in the mitochondrion
Shingomyelin = most common sphingolipid
Found mainly in the liver
*Not all cells have the same lipid composition (varies between cell type and tissue)
Describe the lipid bilayer
basic structure for all cell membranes
Has embedded proteins (50% covered in proteins)
Lipid molecules are amphiphilic (hydrophilic &hydrophobic)
Contains cholesterol and glycolipids
Disordered lipid molecules
Irregular and flexible spacing
Kinks in fatty acid tails allow for more flexibility
Phospholipids are NOT bound to their location
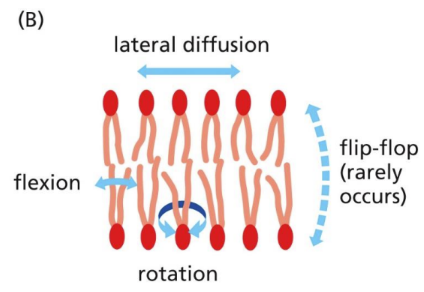
How is the lipid bilayer fluid (un/saturated)
Fluidity = must be maintained for transport and enzymes to work
Lipid bilayer has bent tails (double bond) to make the chains harder to pack together and the bilayer more difficult to freeze thus maintaining functionality at lower temps
Unsaturated: cis-double bonds = freeze resistant, functionality kept
Saturated: no double bonds = freeze at low temps, no movement
What is a lipid raft
patch in lipid bilayer made of specific lipids and proteins
segregate into specialized domains
Small region of a membrane enriched in sphingolipids and cholesterol
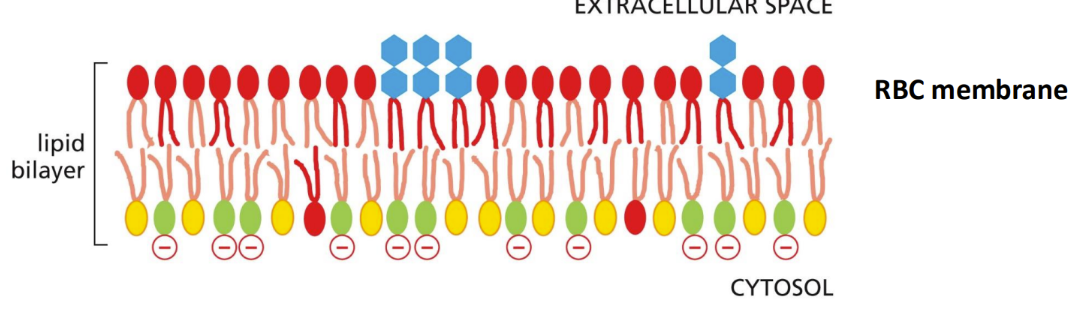
How and why is the lipid bilayer asymmetrical
Asymmetry = crucial for converting extracellular signals into intracellular ones (and for marking dying cells)
Makes charges between the two halves of the bilayer different
Asymmetrical monolayer
Creates electrical chemical gradient
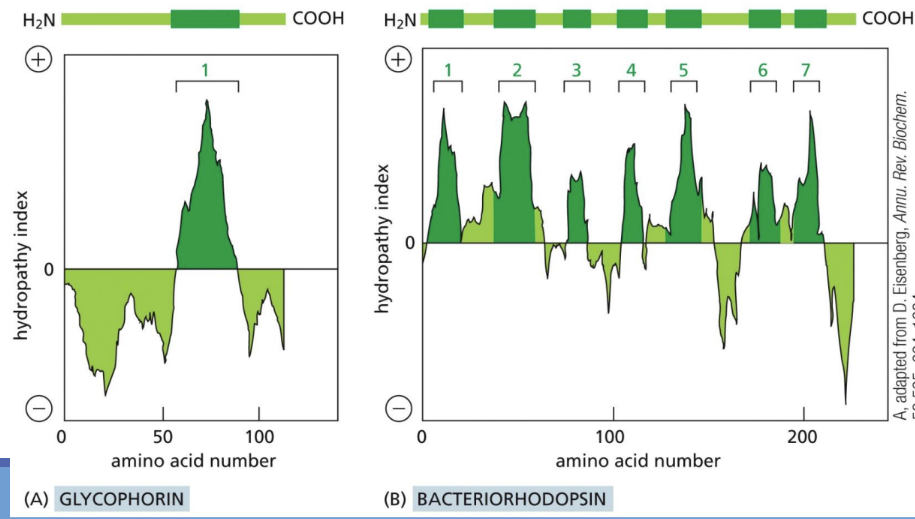
What is a hydrophathy plot
Hydropathy plot = illustrates the number of amino acids of a transmembrane protein located within a cell membrane’s lipid bilayer (~20-30)
How many membrane-spanning domains there are
What are the 3 types of membrane proteins
Types:
Transmembrane
Cytosolic but associate with cytosol side of bilayer
Cell surface but attached to lipid bilayer
What is GPI (glycosylphosphatidylinositol anchor)
special membrane proteins that are actually extracellular made in the ER of the cell as single-pass initially
Transmembrane segment is cleaved and the GPI anchor is added
Anchor is only hold on this protein
Protein is delivered via transport vesicle to the cell membrane
GPI is the anchor
What are peripheral membrane proteins
bound via some noncovalent interaction with other membrane proteins
What are transmembrane proteins
held in the hydrophobic core of the lipid bilayer and CANNOT be released easily
Function on BOTH sides of the bilayer/transport molecules across it
Membrane-spanning domains contact hydrophobic part of lipid bilayer (tails)
Because the inside of the membrane bilayer is hydrophobic, the transmembrane domains are made of non-polar amino acids
Since every peptide bond is polar and no water is present in the bilayer, the amino acids form hydrogen bonds with each other, so peptide chain curls into an alpha-helix as it crosses bilayer
types: single pass, multi pass, and beta barrels
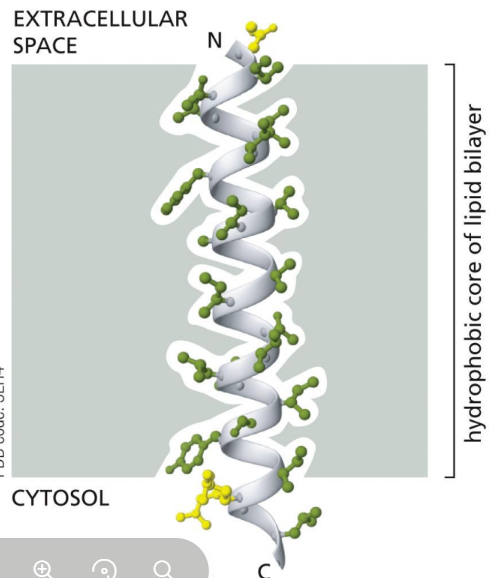
What is a single pass protein
cross the lipid bilayer only once (transmembrane)
What is a multi-pass protein
cross the lipid bilayer multiple times (“porin proteins”)
Can form beta sheets which form into a barrel which is a protein to satisfy hydrogen bonding requirements
More often occur in bacterial membranes and mitochondrial membranes
Most are constructed from alpha-helices that can slide, open and shut, transport or transduce (in eukaryotic cells)
Multiple transmembrane helices can arrange to create channels through the membrane (helices can make channels too)
What are beta barrels
can form channels
Some have projecting amino acid loops into the center, filling the hole
Can function as receptors or enzymes
What is a carbohydrate coat
carbs extensively coat the surface of all eukaryotic cells covalently bound to membrane proteins and lipids
Lectins = carbohydrate-binding proteins
Mediate variety of cell-cell adhesion processes and other recognition routes
Used to study carb coating
What is the eukaryotic cell cortical cytoskeleton
nucleated cells contain a more complex network that makes up the cortical region of the cytoplasm
Cortex = covering
Called the cortical cytoskeletal network = spectrin
Cytoskeletal filaments can be attached to cytosolic membrane and form barriers to membrane protein movement
How and why is the cytoskeleton flexible?
Flexible cytoskeleton = Maintaining shape
Tethering membrane proteins to other proteins inside the cell
EX: RBC = spectrin protein forms cell cytoskeleton, this interacts with membrane proteins to produce a flexible membrane
Hereditary spherocytosis (spectrin mutations) produces RBCs that have unusual cell membrane properties... unflexible, destroyed quickly leads to anemia
What is a intracellular domain
Among the best-characterized plasma membrane proteoglycans are the syndecans, which have a membrane-spanning core protein whose intracellular domain interacts with the actin cytoskeleton and with signaling molecules in the cell cortex
Special cell domains for proteins
Membrane proteins are segregated into specific areas of cell surfaces
Proteins don’t swim through the lipids
Held in place by tight junctions, cytoskeletal components or protein-protein interactions
Keeps proteins and lipids in appropriate places for specific functionality
Protein-protein interactions = keep proteins in separate domains
Self-aggregation
Tethering
Interaction with other cells
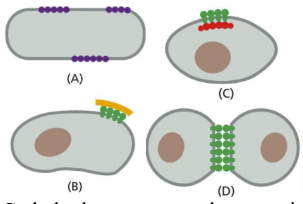
What are extracellular domains
The extracellular domain is linked to multiple GAG chains (primarily heparan sulfate).
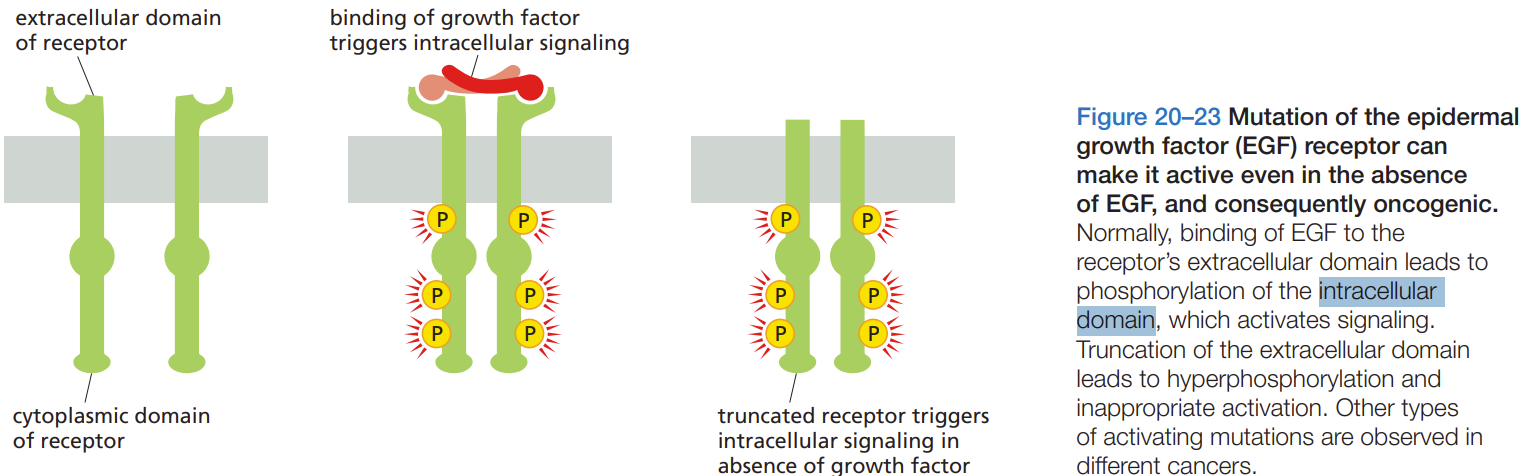
What are some protein-protein interactions
Protein-protein interactions = keep proteins in separate domains
Self-aggregation
Tethering
Interaction with other cells