Biology Chapter 8: Metabolism
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
what is metabolism
the totality of an organism’s chemical reactions
what are some examples of these reactions
synthesizing and hydrolyzing macromolecules
how are reactions that make up cell metabolism characterized?
reactions that make up cell metabolism are complex, efficient, coordinated, and highly responsive to even the most subtle changes
what are metabolic pathways
metabolic pathways begin with specific molecules and end with a product
what are enzymes?
proteins that speed up chemical reactions
what are the two types of metabolic pathways?
anabolic and catabolic
what are catabolic pathways
breaking down complex molecules into simpler compounds
what is an example of a catabolic pathway
cellular respiration
what do catabolic pathways release
energy
what are anabolic pathways
reactions that build complex molecules from simpler molecules
what do anabolic pathways consume
energy
what is an example of an anabolic pathway
polymerization
what is energy
the capacity to cause change
cells transform energy…
from one form to another
what is kinetic energy
energy associated with the relative motion of objects
what is thermal energy
the random motion of atoms
what is potential energy
energy that matter processes due to its location or structure
what is heat
the transfer of thermal energy
what is chemical energy
the potential energy that can be released in a chemical reaction
what is thermodynamics
the study of energy transformations that occur in matter
what is an isolated system
a system unable to exchange energy and matter with its surroundings
what is an open system
a system that can exchange energy with its surroundings
what is an example of an open system
organisms
what is the first law of thermodynamics
energy can be transferred and transformed, not created, nor destroyed
what is the second law of thermodynamics
energy transfer/transformation increases the randomness (entropy) of the universe
what explains the second law of thermodynamics
the fact that energy is always lost as heat
cells, though highly ordered, convert organized energy into heat. this means that organizations…
does not decrease the unstoppable trend towards randomization
a synonym for entropy
randomness
cells create ordered structures from
less ordered materials
cells replace ordered forms with
less ordered forms
what is a spontaneous process
something that occurs without energy input
spontaneous processes increase
the entropy of the universe
non-spontaneous processes
occur without energy input
what is an example of a non spontaneous process
polymerization
what is an example of a spontaneous process
cellular respiration
how is spontaneity quantified
free energy
what is gibbs free energy
energy that can do useful work when temperature and pressure are uniform
as entropy increases, total energy _____________ and free energy ________
remains the same, decreases
in spontaneous processes, the change in G
is negative
in non-spontaneous processes, the change in G
is positive
spontaneous processes
release energy that can be harnessed to do work
in non-spontaneous process
energy is consumed and stored
free energy is a measure of
instability
spontaneous reactions increase
stability by decreasing a system’s free energy
as the cell moves towards equilibrium, it harnesses the energy of _______________
spontaneous processes
exergonic reactions
are spontaneous
endergonic reactions
are nonspontaneous
exergonic reactions release
free energy
endergonic reactions absorb
free energy
the more G released,
the more work can be done
the more G absorbed,
the more work needed to be done
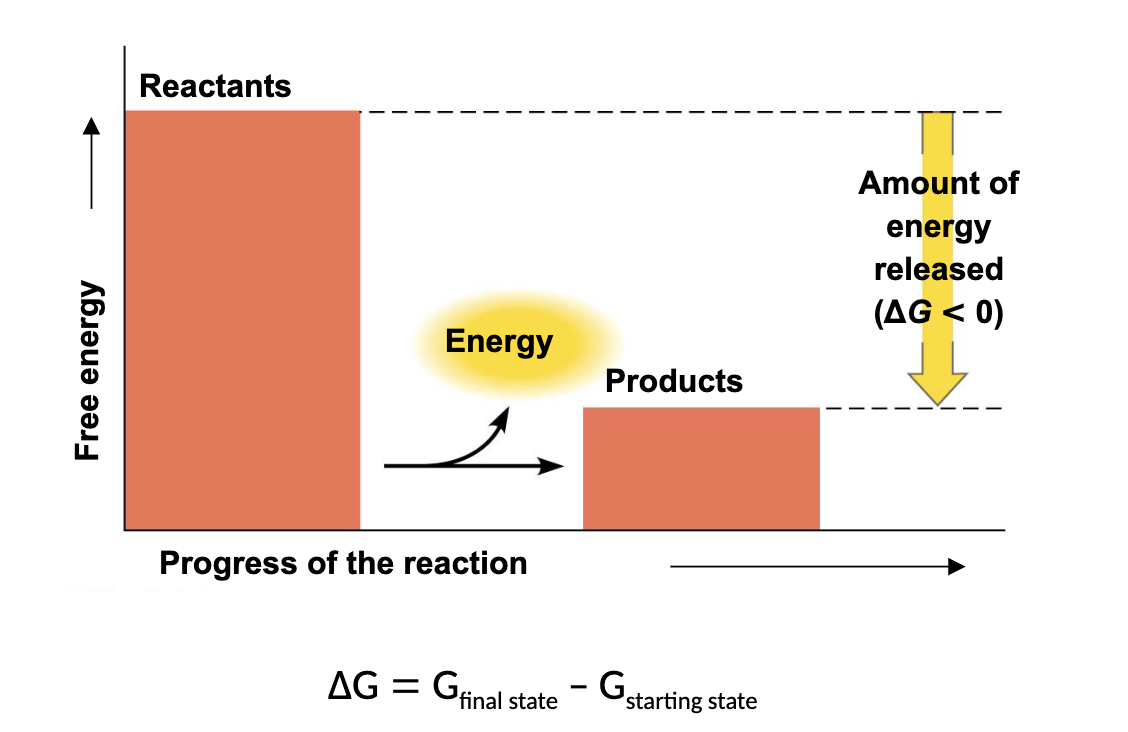
what kind of reaction is this
exergonic reaction
a reaction that has a negative change in G is
exergonic
a reaction that has a positive change in G is
endergonic
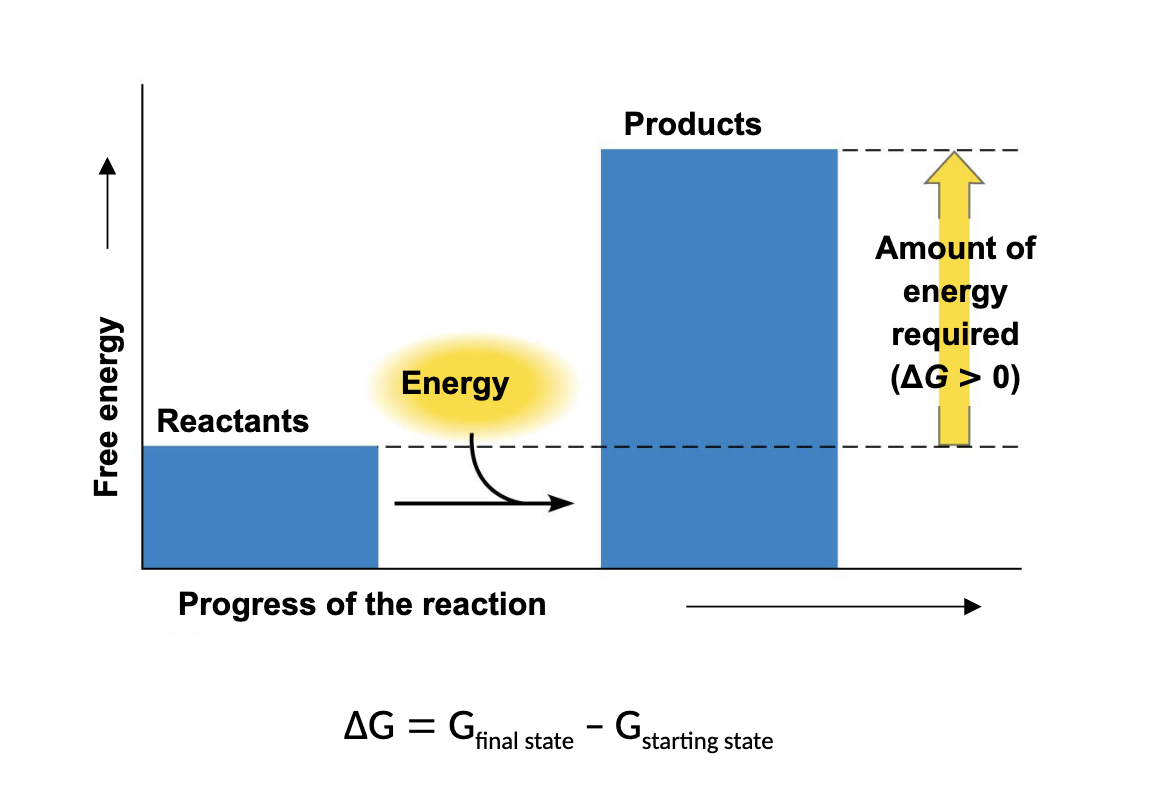
what kind of reaction is this
endergonic
why is metabolic equilibrium equivalent to cell death?
it means the cell is not doing work, and thus, no energy flows into the system
organisms are
open systems
what is a defining feature of life?
metabolic disequilibrium
what is energy coupling
the use of an exergonic process to drive an endergonic reactions
what are the three main kinds of work that a cell does
chemical, transport, and mechanical
chemical reactions are usually
endergonic
what mediates most energy coupling
ATP
what is ATP composed of
ribose, adenine, and three phosphates
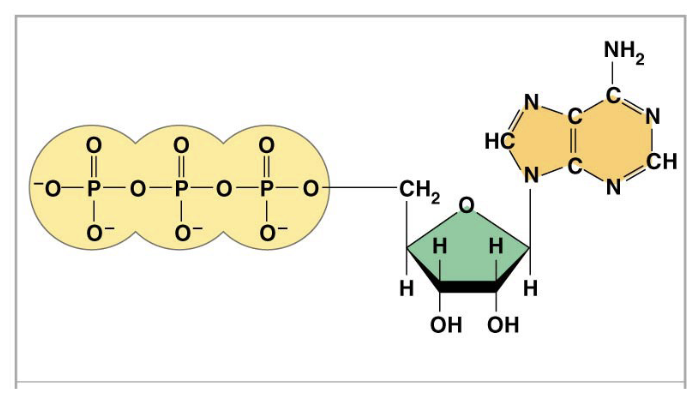
what is pictured here
ATP
what breaks down the bonds between phosphate groups
hydrolysis
what is released when the terminal phosphate bond is broken?
energy
where does this release of energy come from
the energy transformation NOT THE BONDS
why does ATP hydrolysis yield a lot of energy
triphosphate tails are particularly unstable
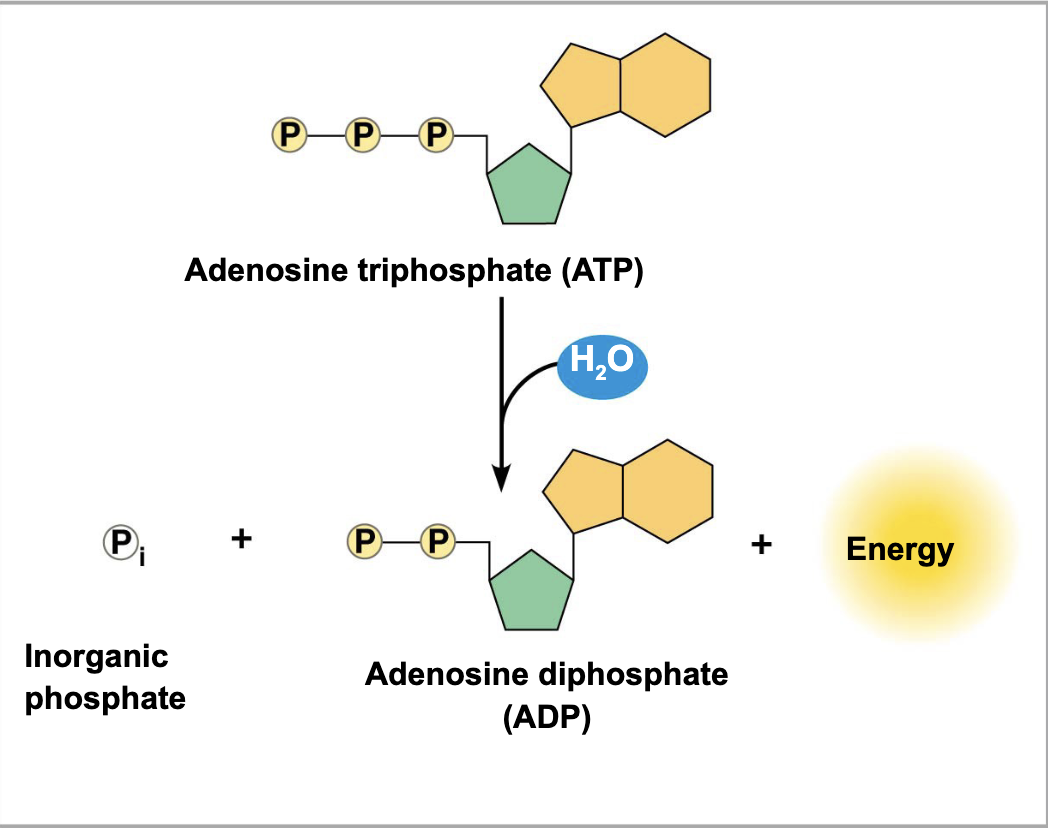
what’s going on here
ATP hydrolysis lols
what kind of work is powered by ATP hydrolysis
chemical work
ATP hydrolysis is
an exergonic reaction
ATP hydrolysis can drive
endergonic reactions
ATP drives endergonic reactions by
phosphorylation
what is phosphorylation
transferring a phosphate group
when a phosphate group is transferred, what is the recipient molecule called?
phosphorylated intermediate
ATP hydrolysis can also power
hydrolysis
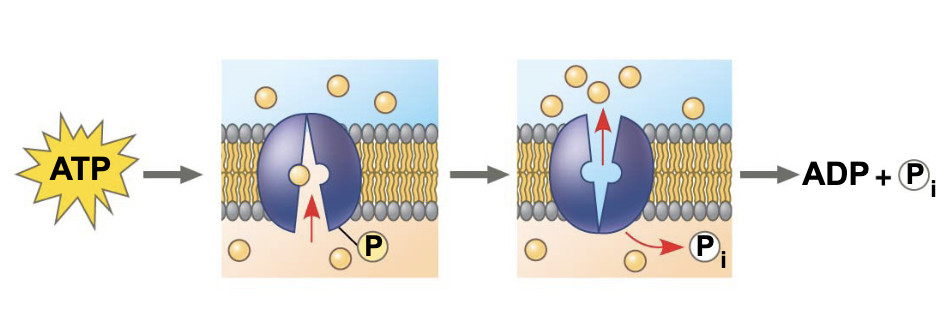
what is going on in this image
ATP phosphorylates transport proteins
ATP hydrolysis can power which kind of protein?
motor proteins
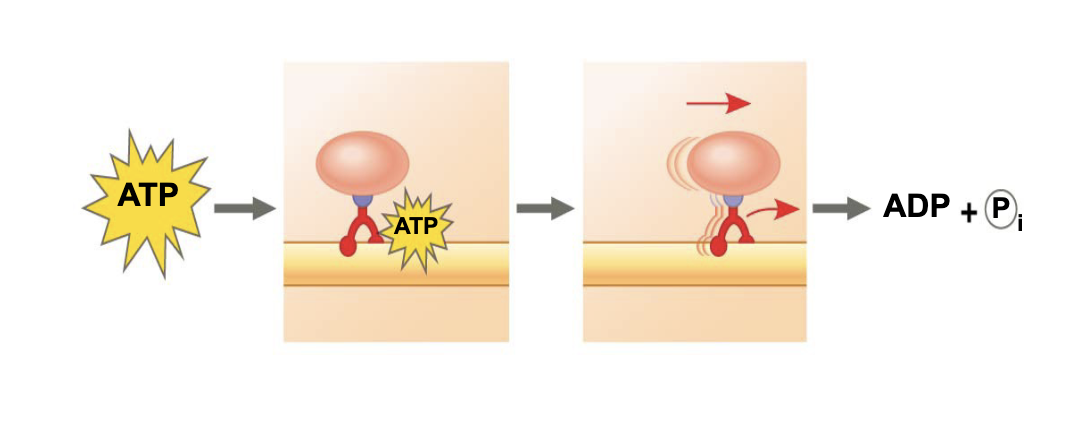
what is ATP doing in this image
powering a motor protein
how is ATP regenerated
by adding a phosphate group to ADP
ATP regeneration is
an endergonic process
catabolic reactions
are exergonic processes
anabolic reactions
are endergonic processes
how do enzymes speed up metabolic reactions
they lower the energy barrier
what is a catalyst
a chemical agent that can speed up a reaction without being consumed by the reaction
chemical reactions involve
bond breaking and forming
what is activation energy
the initial energy needed to start a chemical reaction
how is activation energy usually supplied as
thermal energy absorbed from surroundings
how do enzymes catalyze reactions
they lower the activation energy barrier
do enzymes affect the change in free energy
no, they just speed up reactions that would occur eventualy
enzymes allow for ________ in reactions
specificity and control
what is a substrate
the reactant that an enzyme acts on
why are the reactions catalyzed so specific
because enzymes are proteins which are determined by a unique sequence of amino acids
what is the enzyme-substrate complex
where an enzyme binds to its substrate
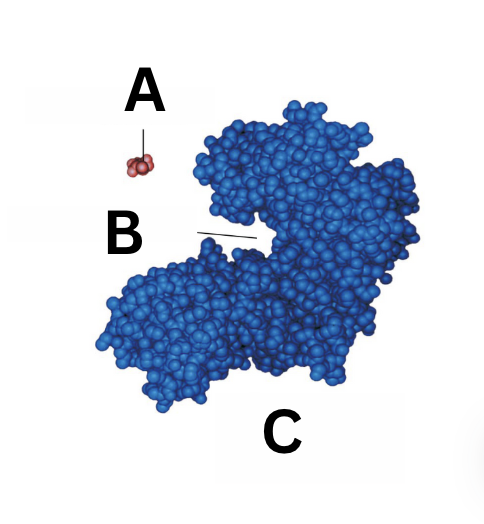
what is A
substrate
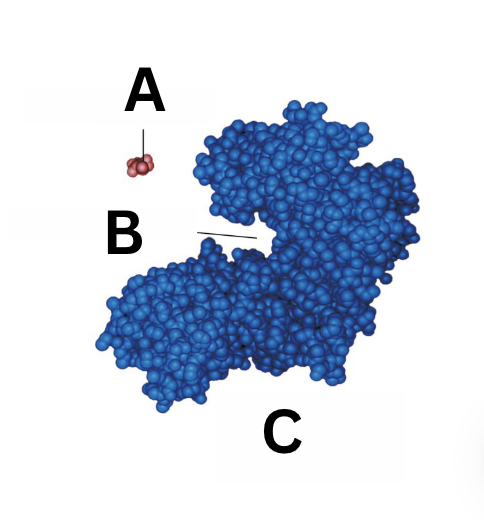
what is B
active site
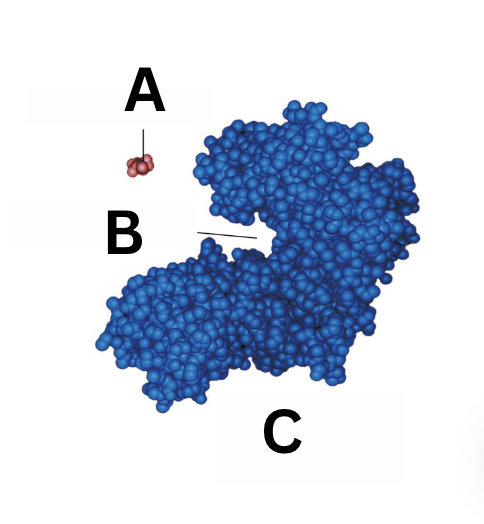
what is C
enzyme
what is the active site
the region where the substrate binds
binding alters
the enzyme’s shape
what is the alteration of the enzyme shape called
induced fit