VSEPR Shapes with angles from workbook
5.0(1)
Card Sorting
1/39
Earn XP
Description and Tags
Last updated 11:27 PM on 11/7/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
1
New cards
Linear (2 domains)
What VSEPR shape has 2 bonding pairs and 0 lone pairs?

2
New cards
Linear (1 domain)
What VSEPR shape has 1 bonding pairs and 0 lone pairs?
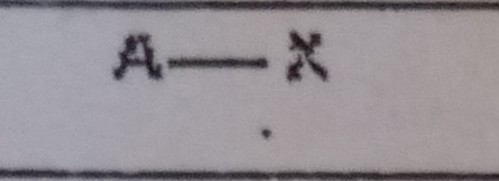
3
New cards
Linear (2 domains)
What VSEPR shape has 1 bonding pairs and 1 lone pairs?

4
New cards
Trigonal Planar (3 domains)
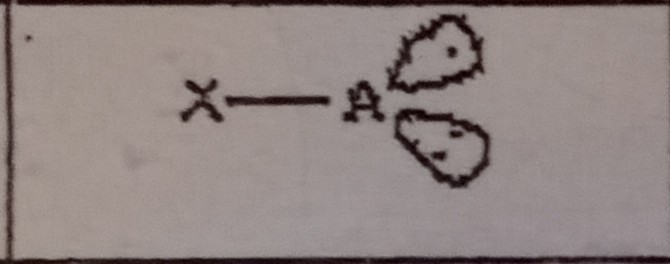
What VSEPR shape has 3 bonding pairs and 0 lone pairs?
5
New cards
Bent @ 120 (3 domains)
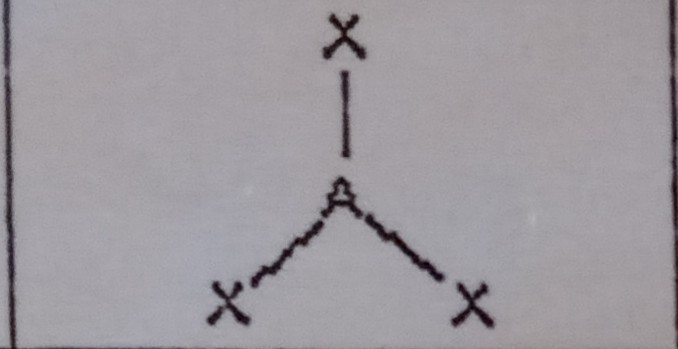
What VSEPR shape has 2 bonding pairs and 1 lone pair?
6
New cards
Linear (3 domains)
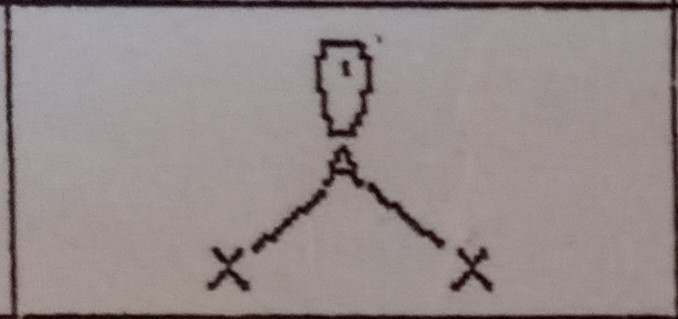
What VSEPR shape has 1 bonding pairs and 2 lone pairs?
7
New cards
Tetrahedral (4 domains)
What VSEPR shape has 4 bonding pairs and 0 lone pairs?
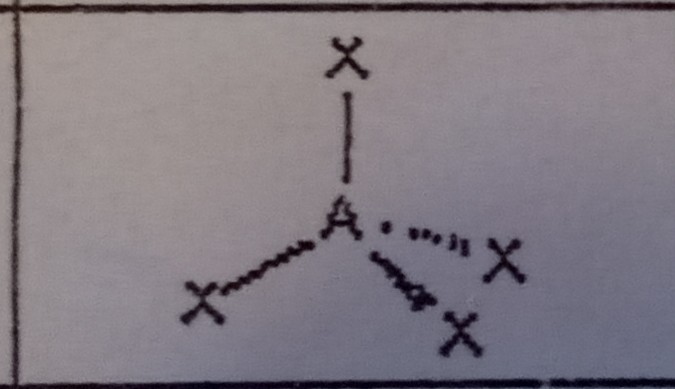
8
New cards
Trigonal Pyramidal (4 domains)
What VSEPR shape has 3 bonding pairs and 1 lone pair?
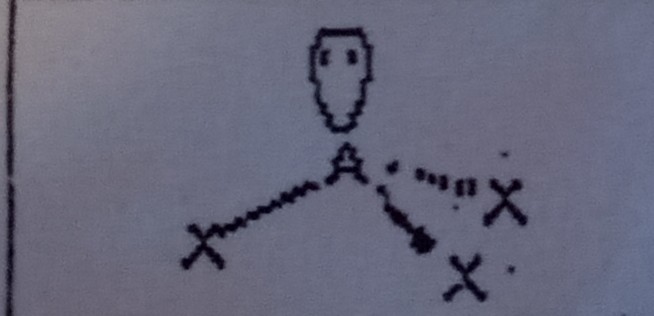
9
New cards
Bent at 109.5 (4 domains)
What VSEPR shape has 2 bonding pairs and 2 lone pairs?
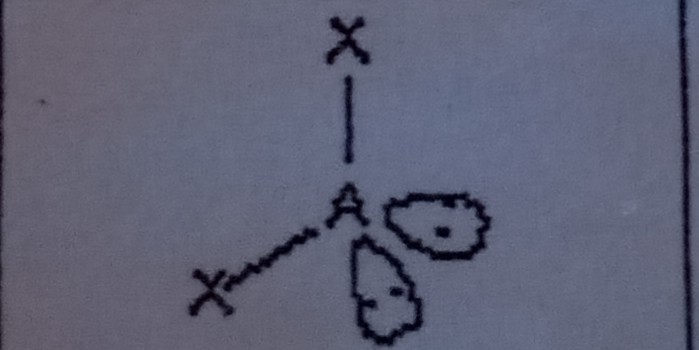
10
New cards
Linear (4 domains)
What VSEPR shape has 1 bonding pairs and 3 lone pairs?
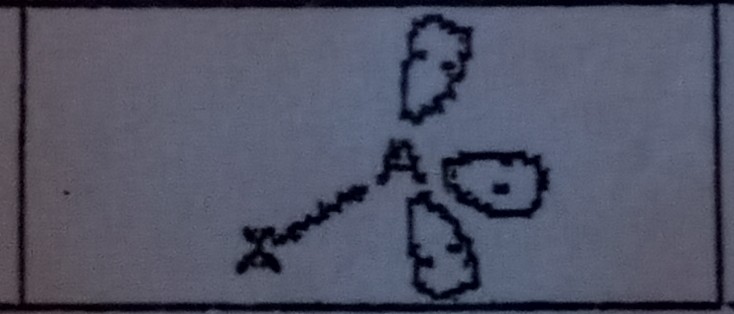
11
New cards
Trigonal Bipyramidal (5 domains)
What VSEPR shape has 5 bonding pairs and 0 lone pairs?
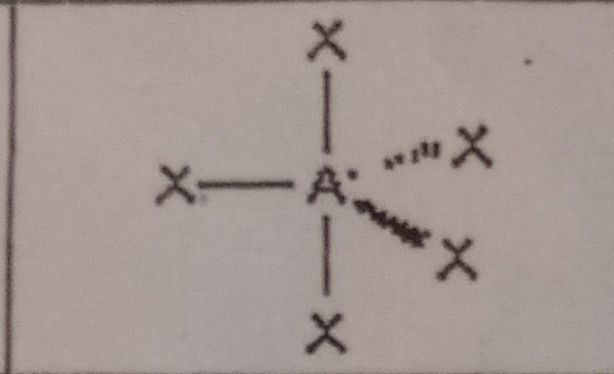
12
New cards
Seesaw (5 domains)
What VSEPR shape has 4 bonding pairs and 1 lone pair?
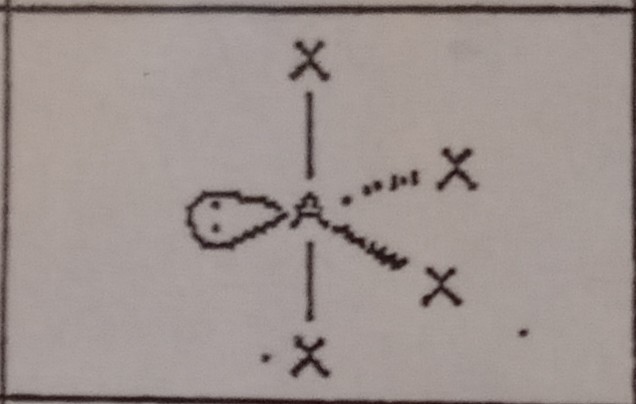
13
New cards
T-shaped (5 domains)
What VSEPR shape has 3 bonding pairs and 2 lone pairs?
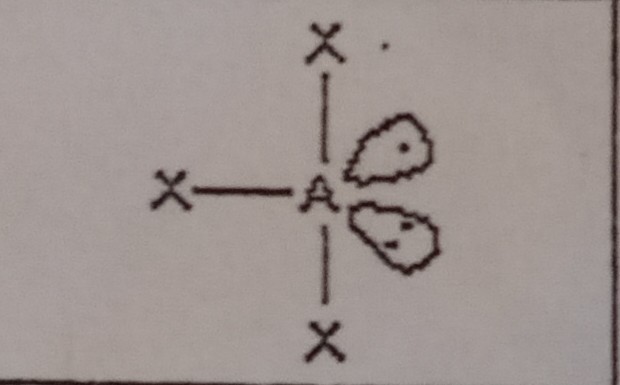
14
New cards
Linear (5 domains)
What VSEPR shape has 2 bonding pairs and 3 lone pairs?
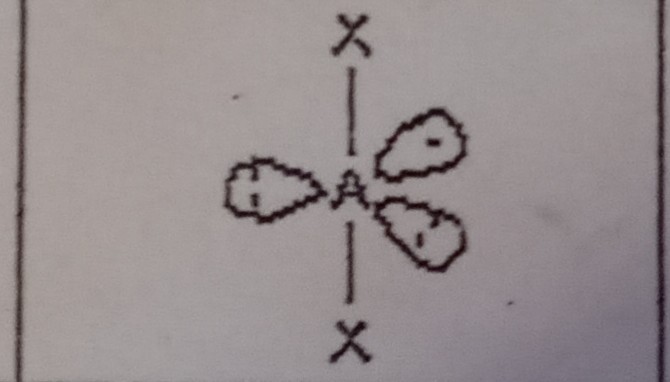
15
New cards
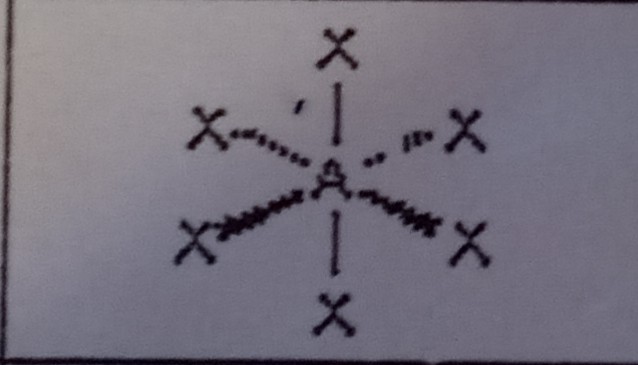
Octahedral (6 domains)
What VSEPR shape has 6 bonding pairs and 0 lone pairs?
16
New cards
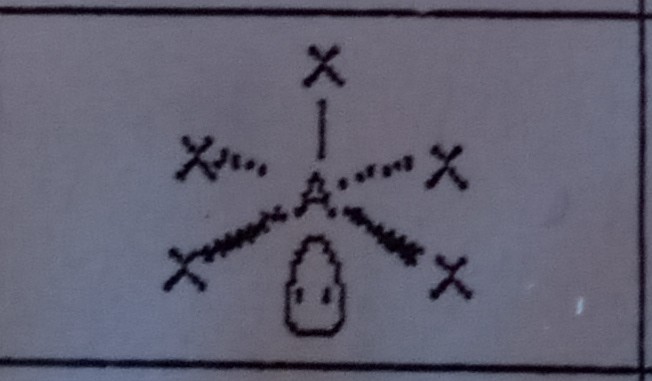
Square Pyramidal (6 domains)
What VSEPR shape has 5 bonding pairs and 1 lone pair?
17
New cards
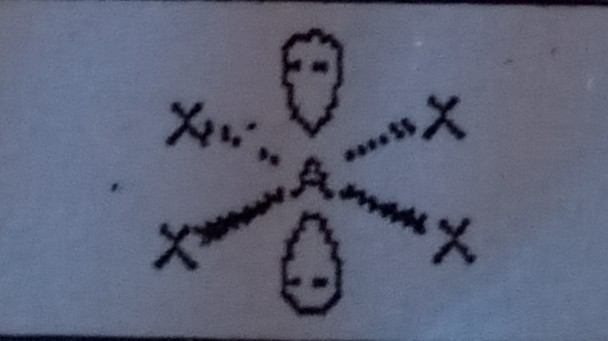
Square Planar (6 domains)
What VSEPR shape has 4 bonding pairs and 2 lone pairs?
18
New cards
180 degrees
What is the bond angle of a linear molecule?

19
New cards
90 < x < 120
What is the bond angle of a bent molecule? (sheet)
20
New cards
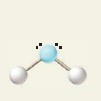
105 degrees
What is the bond angle of a bent molecule?
21
New cards
120 degrees
What is the bond angle of a trigonal planar molecule?
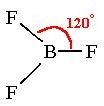
22
New cards
107 degrees
What is the bond angle of a trigonal pyramidal molecule?
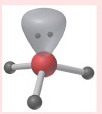
23
New cards
90 & 180 degrees
What is the bond angle of a T-shaped molecule?
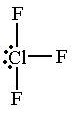
24
New cards
109.5 degrees
What is the bond angle of a tetrahedral molecule?
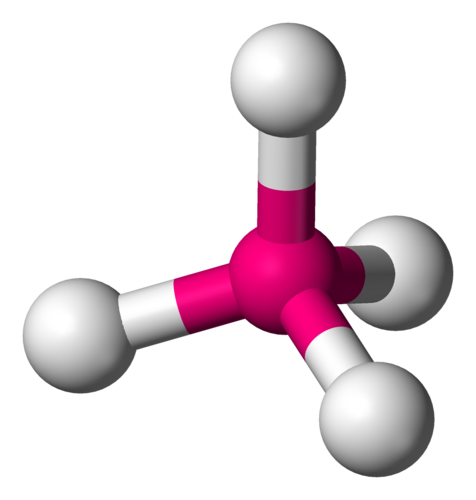
25
New cards
90 < x < 109.5
What is the bond angle of a trigonal pyramid molecule?
26
New cards
90 < x < 109.5
What is the bond angle of a bent molecule (4 domains)?
27
New cards
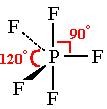
90 & 120 degrees
What is the bond angle of a trigonal bipyramidal molecule?
28
New cards
90 degrees
What is the bond angle of an octahedral, square pyramidal, or square planer molecule?
29
New cards
180
Linear (2 domains) bond angle?
30
New cards
120
trigonal planar bond angle?
31
New cards
x < 120
bent bond angle?
32
New cards
109.5
tetrahedral bond angle?
33
New cards
x < 109.5
trigonal pyramid bond angle?
34
New cards
x < 109.5
Bent (4 domains) bond angle?
35
New cards
120, 90
trigonal bipyramid bond angle?
36
New cards
x < 120, x < 90
Seesaw bond angle?
37
New cards
90
t-shape bond angle?
38
New cards
90
octahedral bond angle?
39
New cards
x < 90
square pyramid bond angle?
40
New cards
90
square planar bond angle?