2.2 Covalent bonding Sl
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What is the octet rule?
Elements will try to achieve a stable electron configuration by having 8 electrons in their valance shells.
The tendency of an atom to achieve stability by ensuring its valence shell is full, either by gaining, losing or sharing electrons
What is a covalent bond?
The electrostatic force of attraction between positive nuclei and a shared pair of electrons.
Electrostatic force of attraction in ionic bonding and covalent bonding
In an ionic bond it is the electrostatic attraction of cations and anions that holds two species together.
In a covalent bond, it is the electrostatic attraction between the positive nuclei of two bonding atoms and their shared pair of negatively charged electrons that holds these atoms together.
What are the limitations of the octet rule?
Steps for drawing the Lewis formula
work out the total number of valance electrons
(If its an ion then add or subtract electrons depending on the charge)
Divide the total number of valance electrons by 2 to see how many bonds there are
Bond the central and peripheral atoms together by drawing a single bond between them.
Assign non-bonding pairs of electrons to the peripheral atoms. (keep going until they achieve noble gas configuration)
Assign any remaining electrons to the central atoms
Check the central atom has a full octet
if it does not then reassign nonbonding pairs on the peripheral atoms to become additional bonds to the central atom
Check the moelcue is not an exception. (Hydrogen and helium)
How to determine which is the central atom in a Lewis structure where there are more than two atoms present?
lowest multiple
Furthest left in the periodic table
(might be the atom written first)
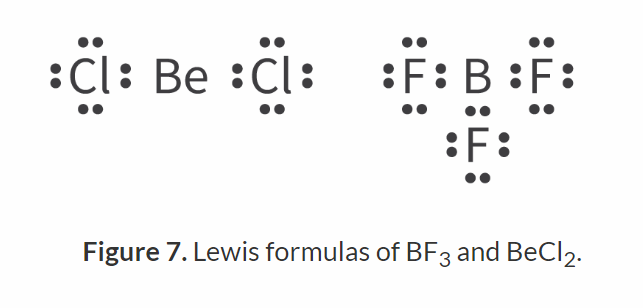
Which metals can form covalent bond? and what compounds can they form?
Beryllium and Boron
Forms BeCl2 and BF3
Whats the difference between organic and inorganic compounds?
Organic compounds:
Must have carbon present and usually contains carbon bonded to hydrogen
Inorganic compounds:
May have carbon present but not bonded to hydrogen
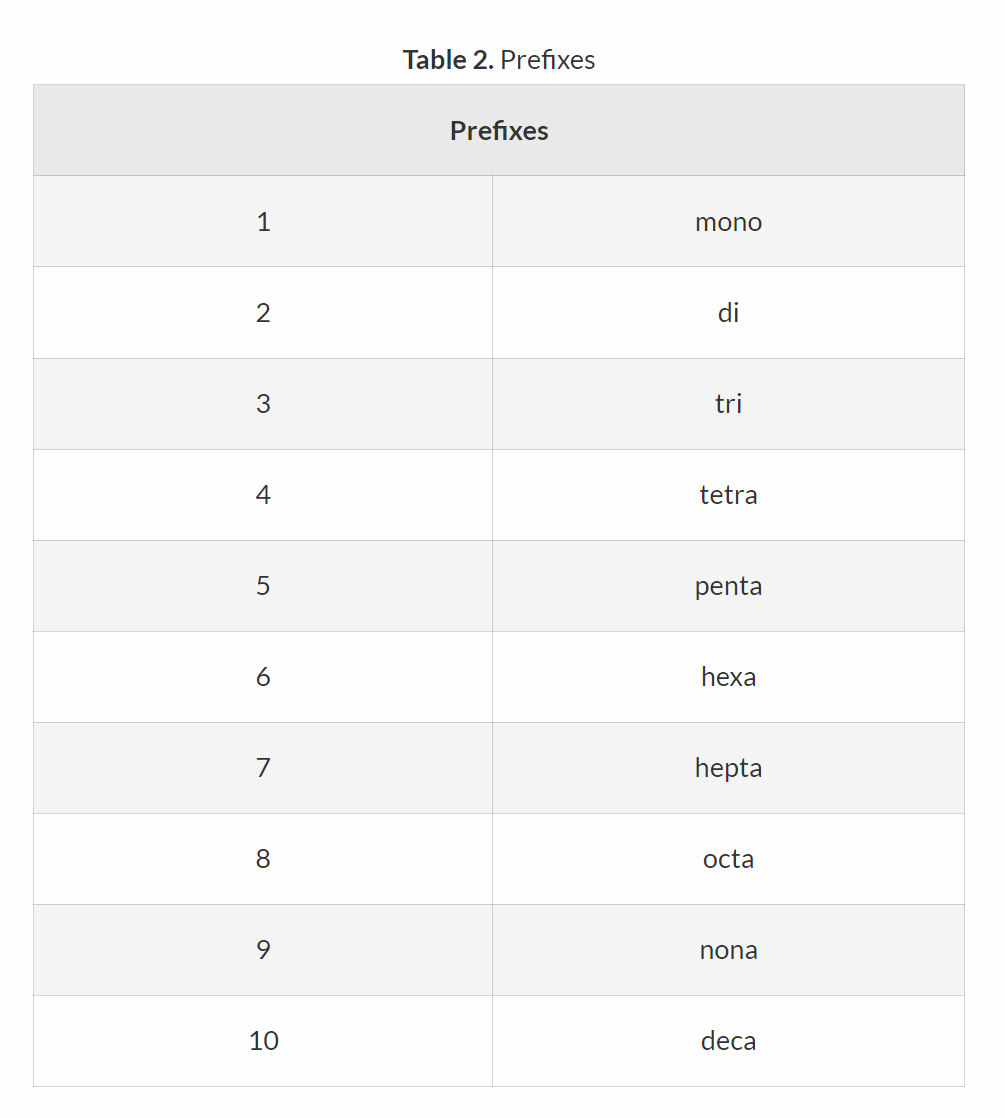
Name all the prefixes until 10 for elements
What is bond strength?
a measure of the energy required to break a bond
Double and triple bonds have a greater bond strength than single bonds, with triple bonds being stronger than double bonds
Multiple bonds involve a greater number of shared electrons so the electrostatic attraction between them and the positive nuclei is stronger, hence a stronger bond.
What is bond length?
a measure of the distance between two bonded nuclei.
Double and triple bonds are shorter than single bonds as the same electrostatic attraction that makes the bond hard to break also pulls the nuclei closer together.
As single bonds only have one pair of electrons holding them together, they are less able to pull the two nuclei together.
What is bond order?
The number of bonds between a pair of atoms
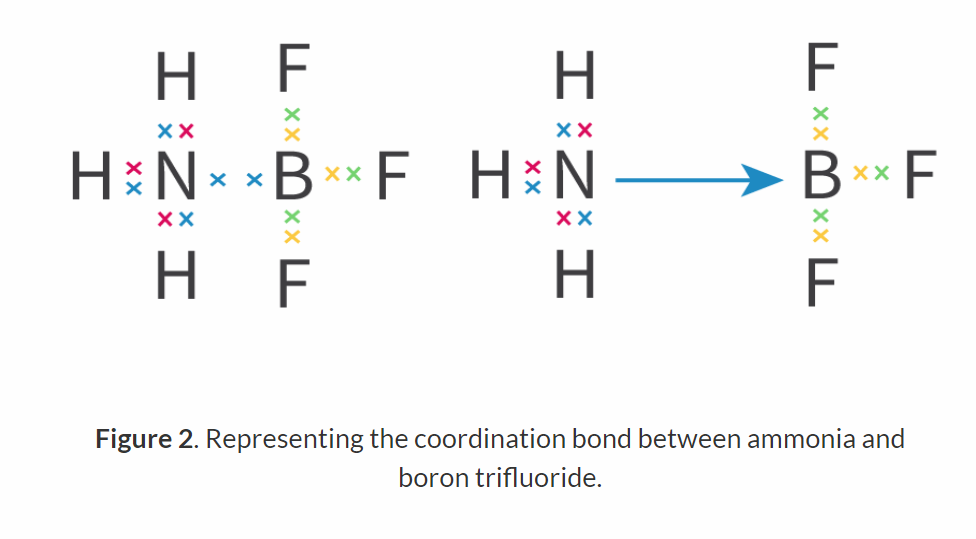
What is coordination bonds?
A covalent bond where both of the electrons being shared in the bond have been donated by one atom.
They consist of 2 electrons, both of which are donated from one atom to the bond.
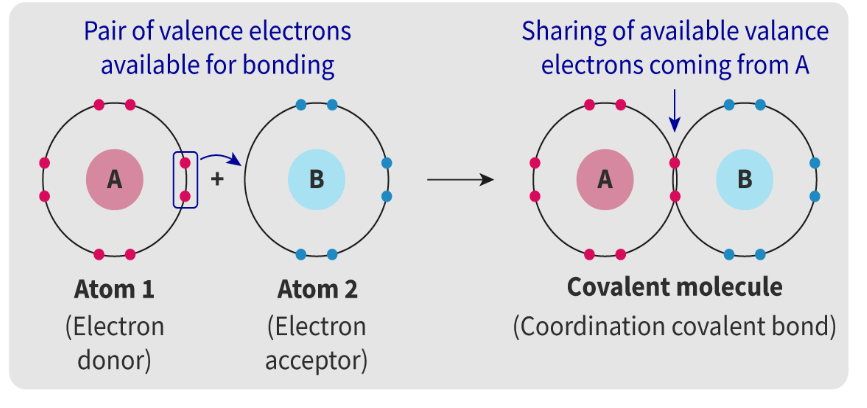
How is coordination bond shown in an Lewis structure?
It is drawn with an arrow. The arrow points towards the electron acceptor originating from the donor.
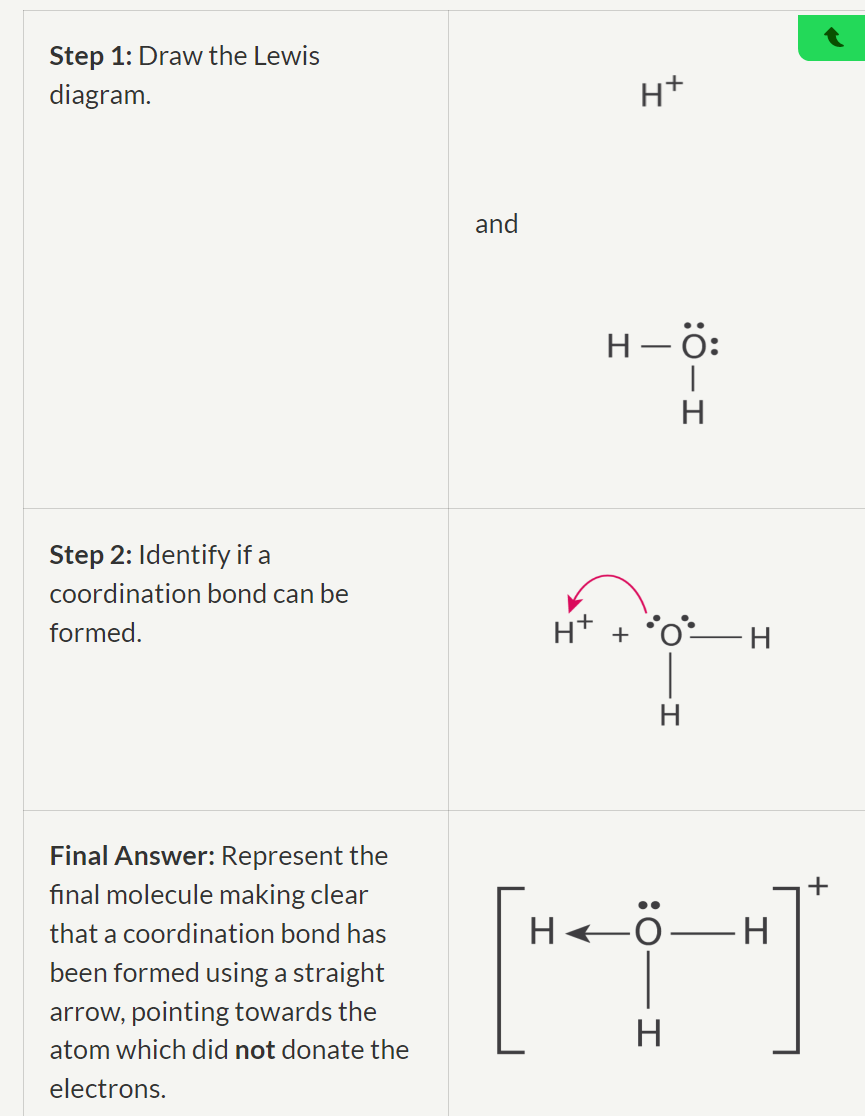
Draw the compound formed from the reaction between a water molecule, H2O and a hydrogen ion, H+.
What is the VSEPR theory?
Valence Shell Electron Pair Repulsion (VSEPR) theory is used to predict the electron domain geometry of the molecule. It is based on the ideas that:
Electron pairs, referred to as electron domains in VSEPR theory, repel each other. Note that it is focused only on the valence electrons of a central atom; electrons in inner-shells are not considered.
An electron domain can be either a single bond, a double bond, a triple bond or a non-bonded pair of electrons. Multiple bonds count as one domain.
non-bonding pairs of electrons cause more repulsion than bonding pairs
Non-polar bonds
A covalent bond formed between two atoms with equal electronegativity values which results in equal sharing of the electrons in their bond.
How does electronegativity affect bond polarity?
Electronegativity is defined as an atom's ability to attract a covalently bonded pair of electrons.
The difference in electronegativity when two or more elements bond together causes an uneven distribution.
The more electronegative atom has a greater attraction towards the shared pair of electrons so it becomes partially negative and the other one becomes partially positive.
The difference between polar and non polar bonds
Polar bonds:
Between atoms with different electronegativity values (typically a difference of 0.4–1.7)
Bonded electrons found closer to one atom than the other atom
Non-Polar bonds:
Between atoms with the same or similar electronegativity values
Bonded electrons found equal distance between two atoms sharing them
When does a polar covalent bond form?
When there is a big difference in electronegativity values of the two atoms being bonded.
a difference in electronegativity values of between 0.4 and 1.7 will result in a polar covalent bond.
one of the atoms has a much greater attraction for the shared electrons than the other atom so they are found closer to one atom than the other.
Because of this, the atom with stronger attraction will have greater electron density than the atom with a lower attraction
Methods of representing polar bonds (Use HCl as an example)
The two separated opposite electric charges, δ+ and δ-, existing in a polar bond in the dipole .
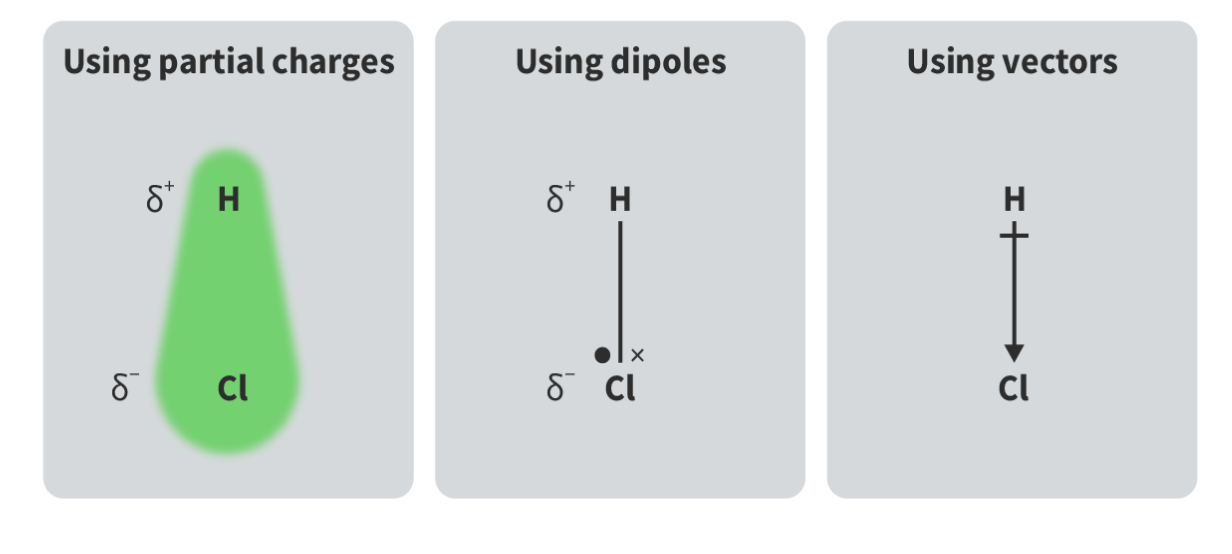
How does the dipole movement determine the polarity of a molecule?
A molecule may have polar bonds present but the symmetry of the molecule may prevent the molecule as a whole being polar if the dipole moments of the individual bonds cancel out
The presence of a net dipole on an asymmetrical molecule means that the positive dipole of one molecule is attracted to the negative dipole of another molecule
All polar molecules contain polar bonds but not all polar bonds result in polar molecules.
Structure of diamond (Bond angle and molecular geometry)
Because each carbon is surrounded by four other carbons, it would form a tetrahedral arrangement with a bond angle of 109.5º
Structure and property of graphite
molecular geometry of each carbon will be trigonal planar with bond angles of 120°.
The carbon atoms form a continuous structure with hexagonal rings, each ring made of six carbons. This makes a planar layer.
each carbon is only bonded to three other carbons hence it contains a delocalised electron which can freely move between the layers in the structure..
The stacked layers in graphite are held together by relatively weak London dispersion forces which makes them easy to separate hence allowing the soft and slippery properties.
Structure and property of graphene
Each planar layer of carbons in graphene is made up of hexagonal rings
bond angles of 120° between the carbons, giving a trigonal planar molecular geometry.
Has strong electrical conductivity and thermal conductivity as there are delocalised electrons present.
Can form nano tubes which are well used in pharmacies ad electrical industries.
Structure of Fullerene
Buckminsterfullerene contains 60 carbons.
Fixed number not a giant network. so simple molecule not a macro molecule
each carbon in fullerene is bonded to three other carbons.
trigonal planar molecular geometry and a bond angle of 120°.
These form a mix of 20 hexagonal (made of 6 carbons) rings and 12 pentagonal (made of 5 carbons) rings
delocalised electrons present in fullerene. However, its conductivity is much poorer than that of graphite. because they can only move around each fullerene and not jump between two separate fullerenes limits their conductivity because they cannot move in along a linear path as with graphite and graphene.
Both nanotubes and fullerenes have wide uses in the medical industry due to their ability to bind to specific target molecules and to carry specific drug and gene therapies to a target site
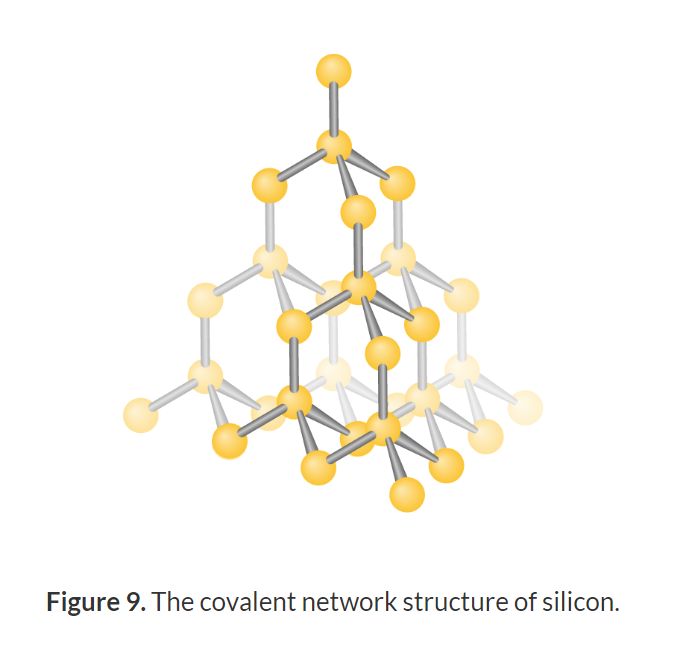
Structure and property of Silicon
Pure silicon has a covalent network structure with each central silicon atom having a tetrahedral molecular geometry and a bond angle of 109.5°.
Strong bonds so high melting and boiling points
Group 14 so 4 valance electrons hence can form 4 covalent bonds with itself and as all the valance electrons are involved in bonding, its electrical conductivity is poor.
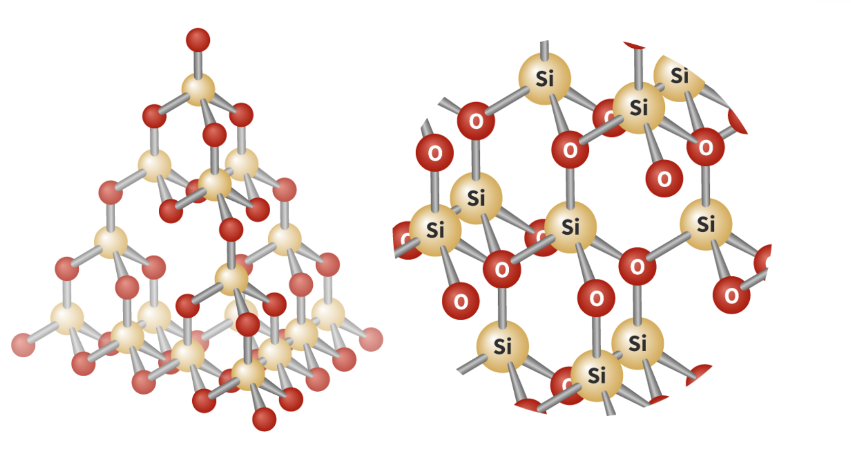
Structure and property of silicon dioxide
SiO2 (empirical formula)= silicon is bonded to 4 oxygen atoms with each oxygen also bonded to 2 silicon atoms.
Tetrahedral geometry with bond angles of 109.5.
high melting boiling points as strong covalent bonds so significant amount of energy is required to break the bonds.
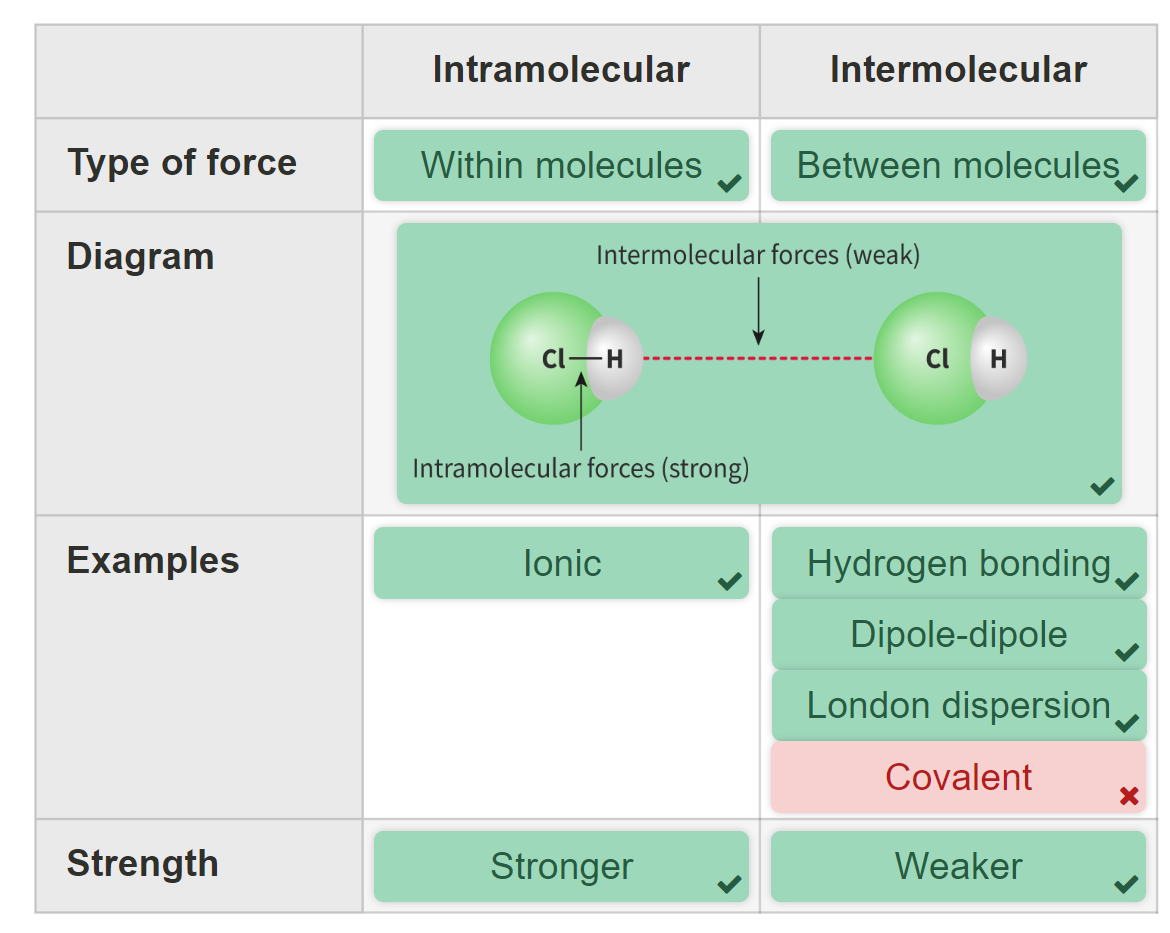
What’s intra molecular forces?
The covalent bonds between atoms within a molecule.
What’s the difference between intra and inter molecular forces?
As these bonds occur within the molecule, these forces are known as an intramolecular, meaning within the molecule.
Intermolecular forces are forces of attraction between two adjacent molecules.
The forces acting between discrete molecules of bromine are intermolecular forces and they are responsible for the physical state of matter of covalent substances, i.e. if they are solid, liquid or gas. (depends on the polarity)
It is the intermolecular forces that must be overcome, not the intramolecular forces. Intramolecular forces are much stronger than intermolecular forces.
Types of intermolecular forces
London dispersion
Dipole-induced dipole
Dipole–dipole
Hydrogen bonding
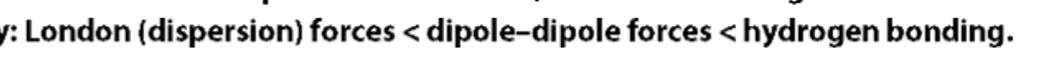
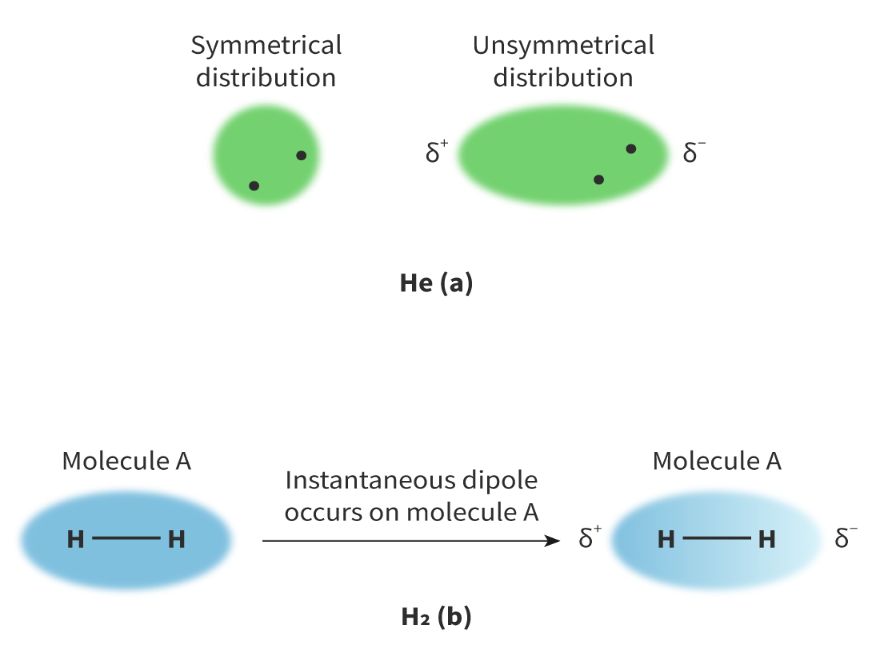
London dispersion forces
the random, continuous movement of electrons can create asymmetrical electron distribution which causes attraction between neighbouring atoms or molecules.
London dispersion forces exist between all molecules, regardless of whether the electronegativity difference is very low or non-existent and even if other forces are also acting. They are the weakest type of intermolecular force.
Dipole-Dipole intermolecular force
if polar bonds are not arranged symmetrically in a molecule then the molecule will have a permanent dipole.
the opposite partial charges on the molecules are attracted to each other.
This type of force will only exist between two asymmetrical molecules that each have a permanent dipole. Dipole–dipole forces are much stronger than London dispersion forces, but can act in unison with them
Dipole-Induced-Dipole
Dipole–dipole forces exist in asymmetrical molecules that have a permanent dipole.
The partial positive on one molecule attracts the partial negative on another molecule and vice versa.
The forces of attraction when a polar molecule induces a dipole in an atom or a non polar molecule by disturbing the arrangement electrons in the non-polar species .
Explains why the boiling points of the halogens increase as their molecular masses increase
As molecular mass increases, the number of electrons increases.
This increases the probability that their electron density can become temporarily unsymmetrical,
causing a temporary dipole.
The intermolecular attraction due to temporarily induced dipoles increases .
What is hydrogen bonding and what are the requirements for hydrogen bonding?
The intermolecular attraction between two molecules which both contain a hydrogen bonded to a highly electronegative element such as oxygen, fluorine or nitrogen.
Hydrogen bonded to a FON element.
The FON element must have a non-bonding pair of electrons.
The hydrogen bond is the attraction for the ẟ+ hydrogen and the non-bonding pair of electrons
Warning:
The hydrogen bond is not the intramolecular bond that exists between hydrogen and the FON in the same molecule.
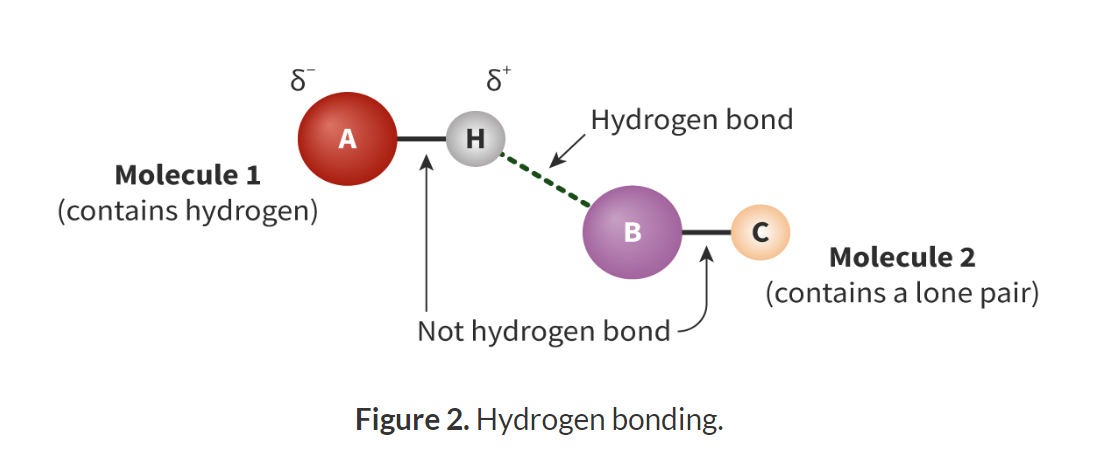
What is the cause of the strong dipole in a FON element?
significant electronegativity difference between the elements.
allows the hydrogen to have a strong ẟ+.
strength of this ẟ+ is increased by the fact that hydrogen has no inner electrons to shield the impact of its nucleus.
This makes the ẟ+ hydrogen very attracted to non-bonding pairs of electrons on neighbouring molecules.
strongest intermolecular force
Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
Explain the trend in the boiling points of HCl, HBr and HI.
(HF has hydrogen bonds between molecules which is the strongest intermolecular force. More energy (hence higher boiling point) is required to overcome this strong force.
HCl, HBr and HI do not experience hydrogen bonds or dipole–dipole. All of these molecules will experience London dispersion forces and as you progress down the period from HCl to HI the molecular size (i.e. number of electrons) on the halogen will increase. This causes the strength of the London dispersion force to increase.
Type of force
Molecular size
How does the type of force affect the strength of force?
How does the molecular size affect the strength of force?
As the molecular size of the compound increases, so does the strength of the force it increases.
The greater the number of electrons in a molecule, the greater the probability of electron distribution asymmetry and the more susceptible the molecule is to developing an induced molecule from a charge nearby.
As the strength of the attraction is greater, so is the energy required to overcome it.