Chemistry- Classification of matter
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Groups

Atomic radius
Distance from the nucleus to the outermost electron shell of an atom
Decreases across a period
Increases down a group
Ionic radius
The distance from the nucleus to the outermost electron shell of an ion
Decreases across a period
Increases down a group
Parent ions
Cations are smaller than parent ions (due to increased effcetive nuclear energy pulling electron closer)
Anions are larger than parent ions (due to extra repulsion spreading the elctron cloud out further)
Ionisations energy
Increases across a period
Decreases down a group
Electron affinity
Amount of energy released when one mole of electrons is gained by one mole of atoms of an element in the gaeous state to form one mole of gaseous ions.
1st is usually exothermic/ 2nd can be endothermic
Exothermic across a period
Endothermic down a group
Electronegativity
Increases across a period
Decreases down a group
Physical Group 1 properties
Softer as you go down the group
Conduct heat and electricity
Have low melting points and densities
Chemical Group 1 properties
Reactivity increases down a group

Colours and states of halogens at room temp
Colour of halogen solutions in water:
Fluorine: not typically tested due to its reactivity
Chlorine: green-blue solution
Bromine: orange solution
Iodine: dark brown solution

Reactivity trend in group 17
Decreases down the group
Displacement
Chlorine>Bromine>Iodine

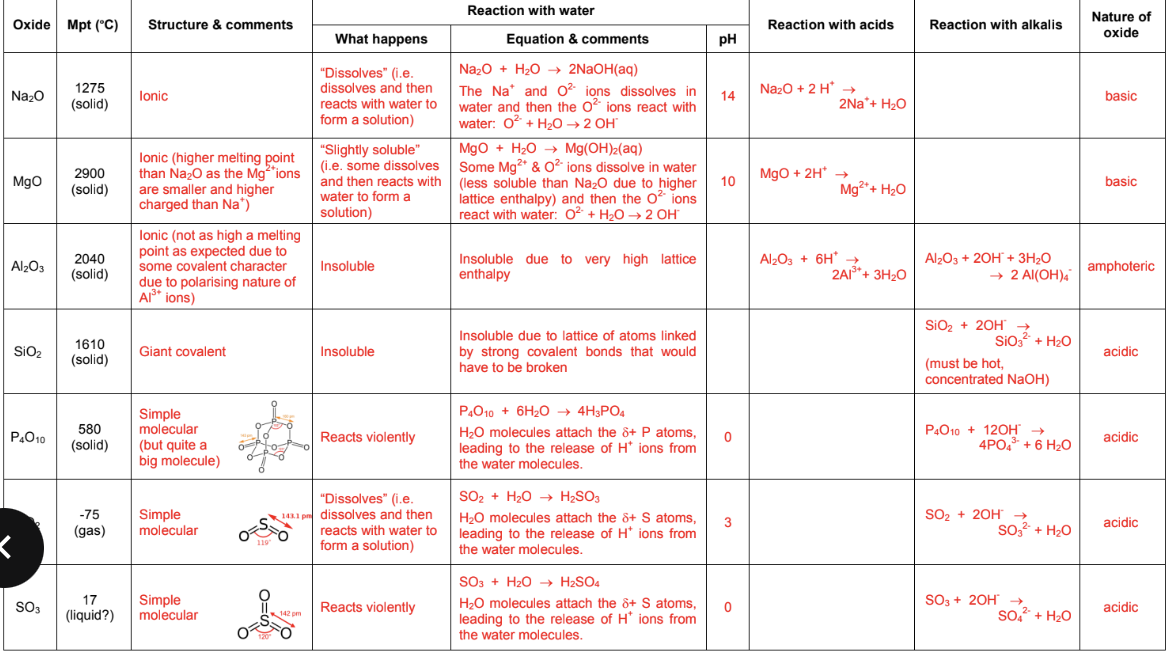
Period 3 oxides
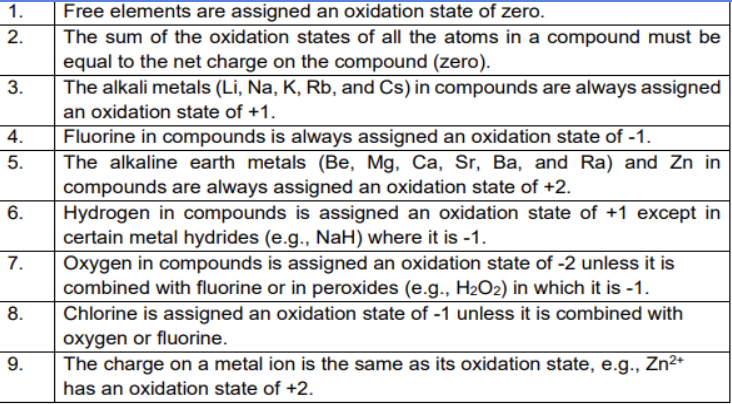
Oxidation states
Absorbed vs. Transmitted colour
The colour that is seen is complementary to the colour that is absorbed