Families/Groups of the periodic table & Properties of the focused groups
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
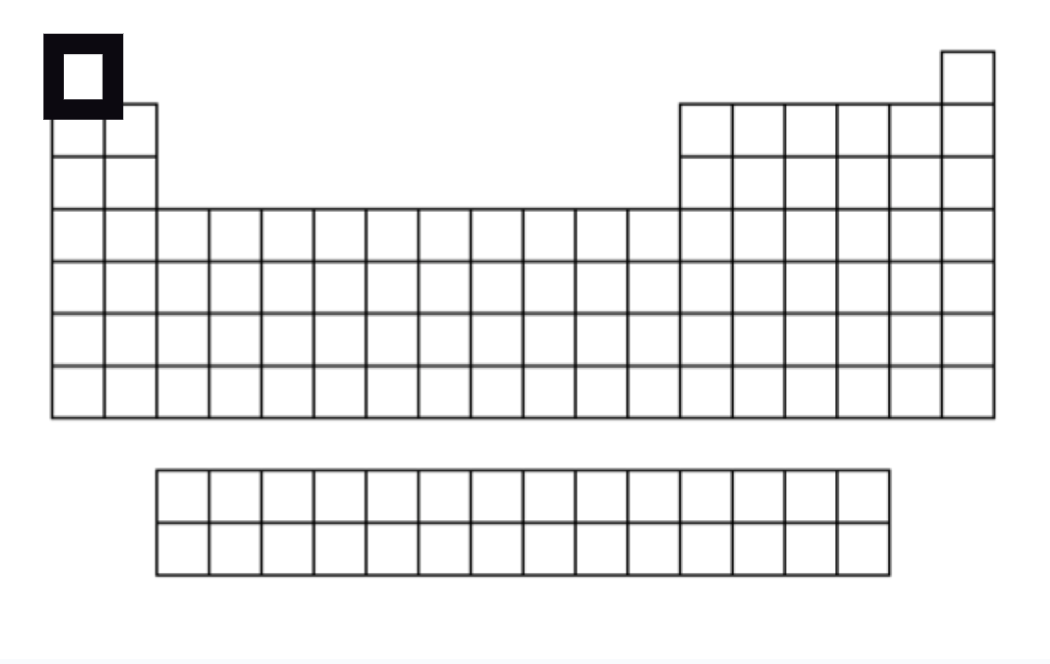
Hydrogen
Is an exception - in Group 1, but is NOT part of that family.
Gas at room temperature.
1 proton, 1 electron & 0 neutrons in its 1 energy level
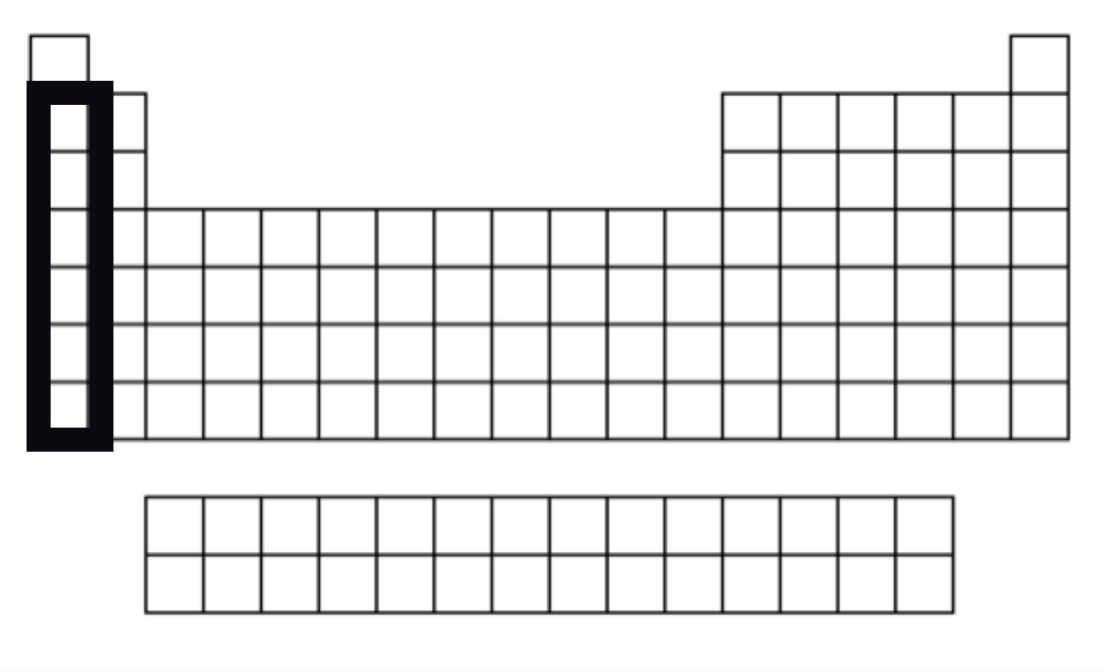
Alkali Metals
Group 1
1 VSE
Shiny, soft & have the consistency of clay
React violently with water and so are never found free in nature.
Most reactive metals
They give away their valence electrons to other atoms.
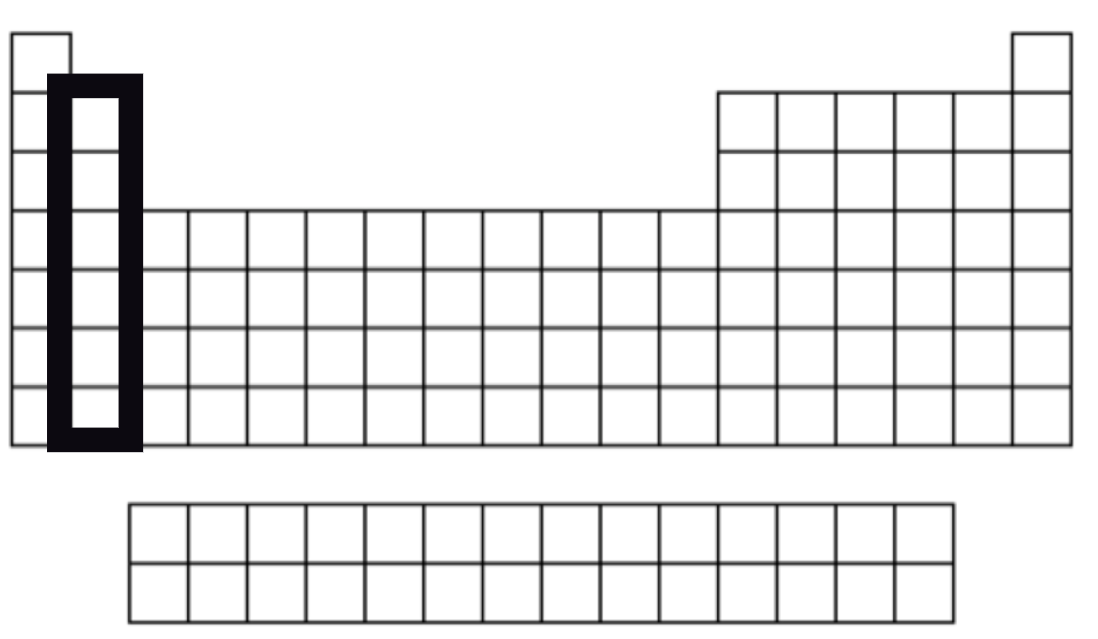
Alkaline Earth Metals
Group 2
Very reactive but not as reactive as group 1
2 valence electrons.
They give away their valence electrona to other atoms.
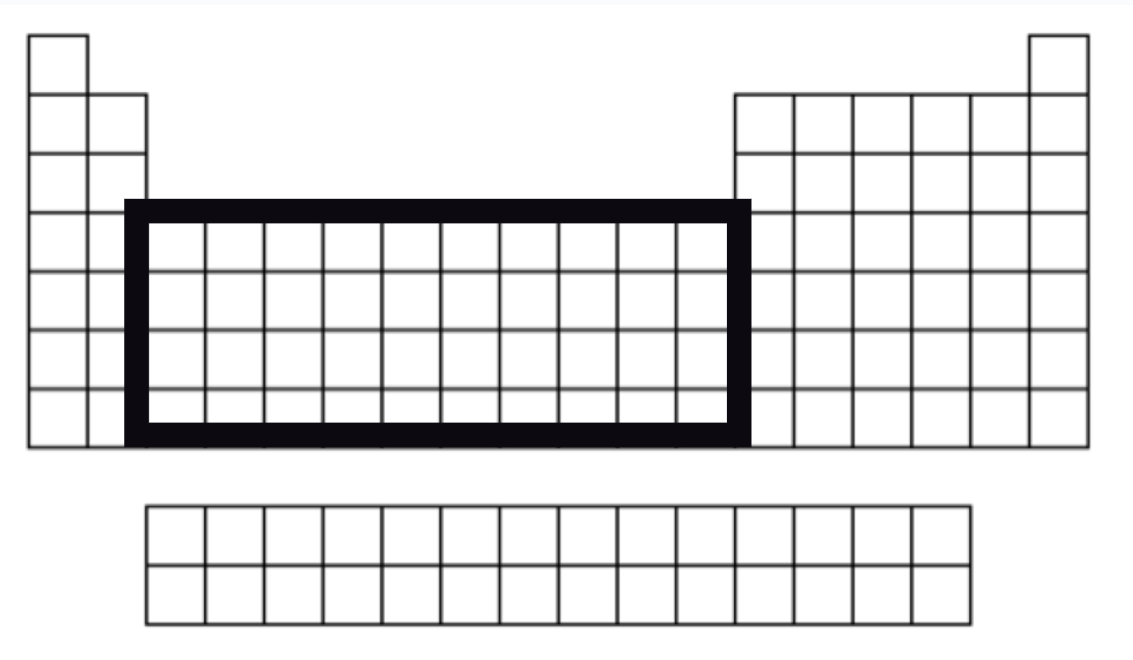
Transition Metals
Number of VSE varies
Good conductors of heat and electricity
Groups 3-12
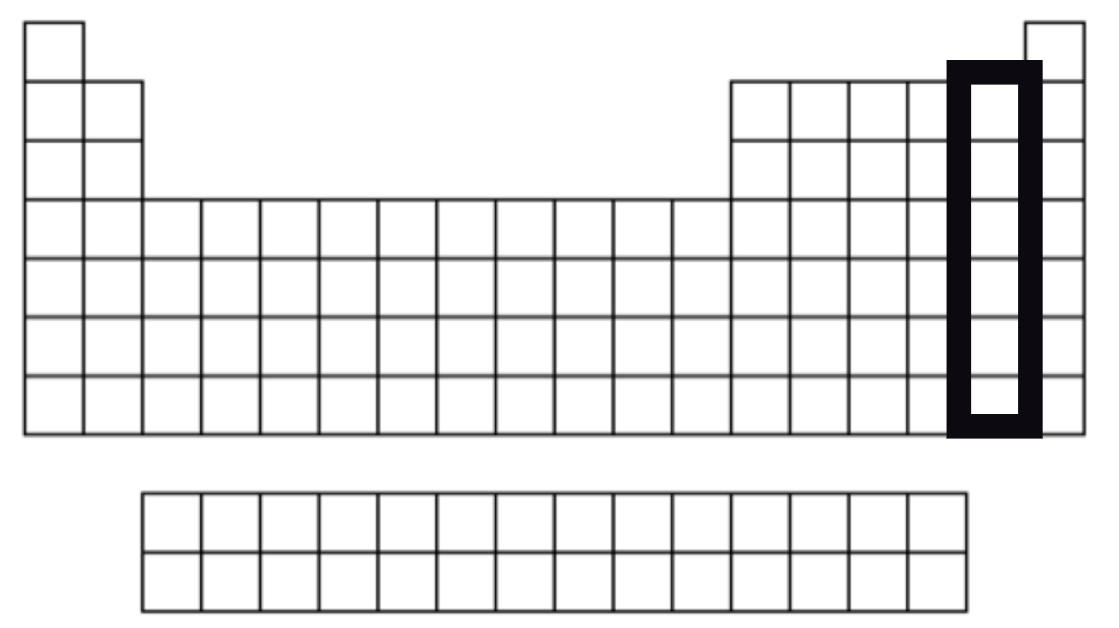
Halogen Family
7 VSE
Very reactive therefore they are never found free in nature
Non-metals
Group 17
Dull
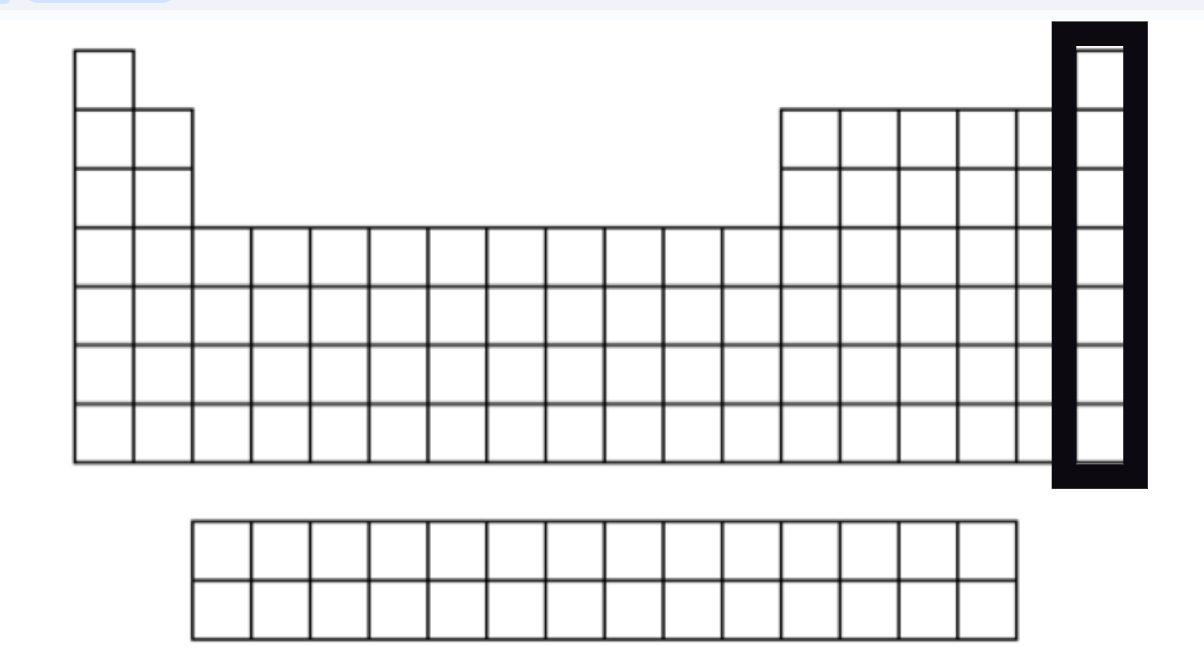
Noble Gases
Gasses
Colorless
Extremely unreactive (inert)
Stable - full octet (8 VSE) - except He (2 VSE)
Found in small amounts in earth's atmosphere.
Group 18
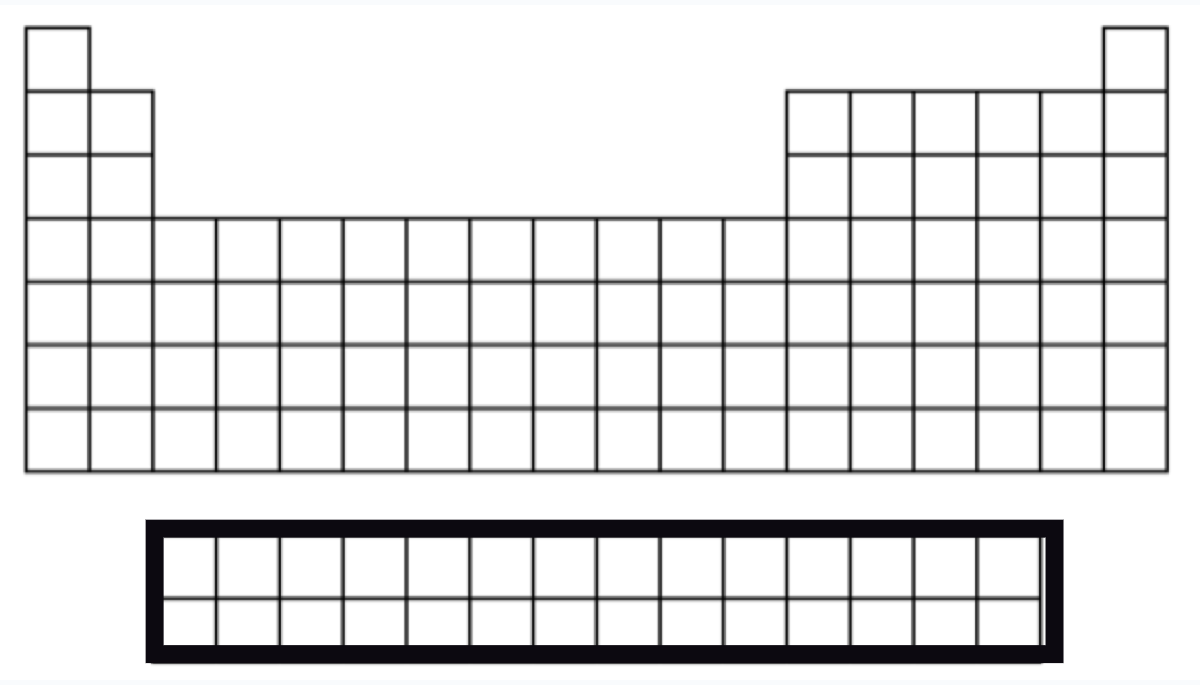
Rare Earth Elements (Metals)
30 → lanthanide and actinide series
Most are synthetic or man-made
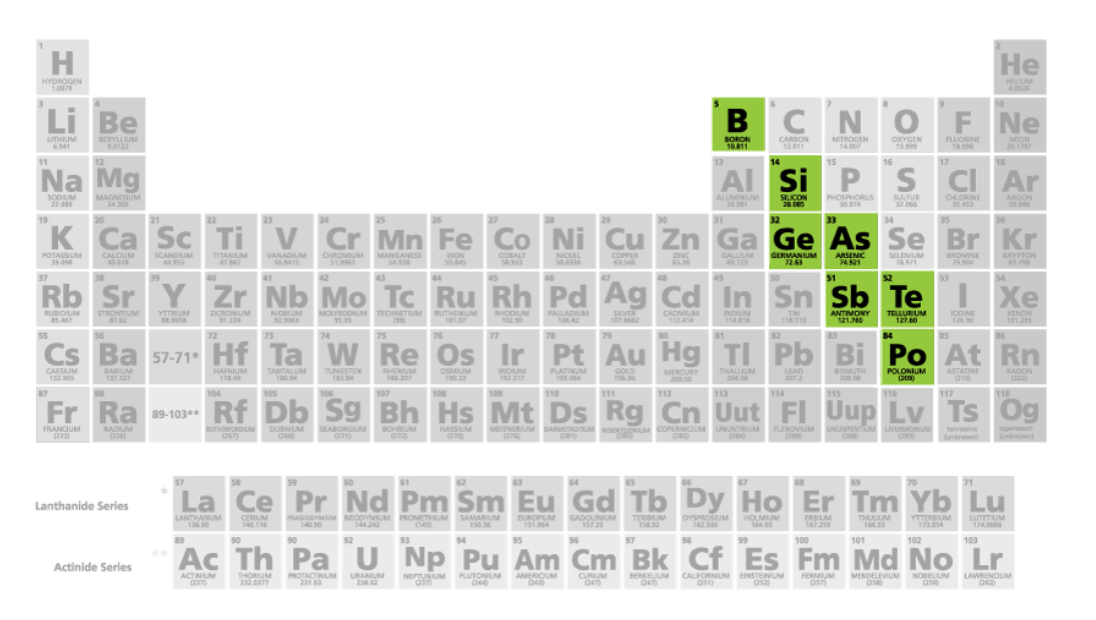
Metalloids
Metal-like
Has properties of both metals and nonmetals
Solids
Shiny or dull
Conduct heat and electricity better than non-metals but not as well as metals
Not ductile/malleable
Elements: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium