Astrobio 1
0.0(0)
Card Sorting
1/51
There's no tags or description
Looks like no tags are added yet.
Last updated 4:45 AM on 9/29/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
1
New cards
Why Carbon (3)
1) makes polymers
2) does not go in rocks
3) becomes atmosphere
2) does not go in rocks
3) becomes atmosphere
2
New cards
Why water
1) large liquid zone (H bonds)
2) volatile
3) abundant (h and O like to bond)
2) volatile
3) abundant (h and O like to bond)
3
New cards
Drake Equation
N = R fp ne fl fl fc L
4
New cards
origin of life heterotrough
cold
eat organic ocean
eat organic ocean
5
New cards
Autotrophic orign
hydrothermal vent
methanogenesis
methanogenesis
6
New cards
metabolism first
cycles of chemical reactions --> metabolism -> genetic molecules
7
New cards
RNA first
Genetic soup -> genetic molecule -> RNA -> DNA/protien
8
New cards
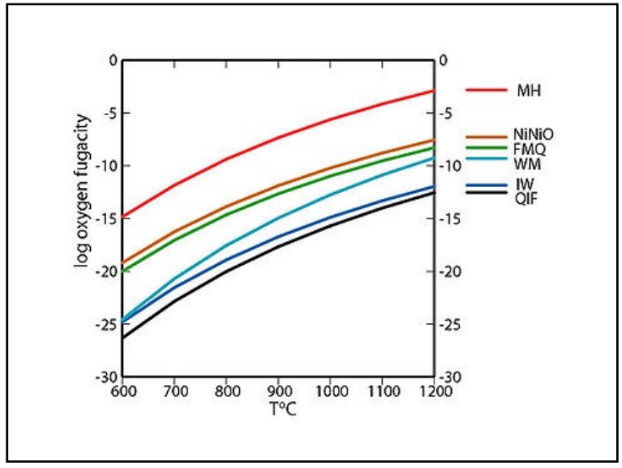
IW redox buffer
Iron Winstite
Fe + O2 --> FeO
Fe2+ --> Fe2+
very reduced
moon/mars
Fe + O2 --> FeO
Fe2+ --> Fe2+
very reduced
moon/mars
9
New cards
FMQ redox buffer
Faylite Magnetite Quartz
3Fe2SiO4 + O2 --> 2Fe3O4 + 3SiO2
Fe3+ --> Fe3+, Fe2+
middle reduced
Today's Earth
3Fe2SiO4 + O2 --> 2Fe3O4 + 3SiO2
Fe3+ --> Fe3+, Fe2+
middle reduced
Today's Earth
10
New cards
PPM redox buffer
pyrrhotite, Pyrite, Magnetite
6FeS + 2O2 --> 3FeS2 + Fe3O4
oxidized
crust/ocean/hydrothermal vents/early earth
6FeS + 2O2 --> 3FeS2 + Fe3O4
oxidized
crust/ocean/hydrothermal vents/early earth
11
New cards
DNA decay
Cytosine --> uracil
The nitrogen carbon bond gets hydrolysed
1/2 life 17,000 years
The nitrogen carbon bond gets hydrolysed
1/2 life 17,000 years
12
New cards
Nucleic bases
Cytosine
Uracil
Guanine
Thymine
Adenine
Uracil
Guanine
Thymine
Adenine
13
New cards
Purines
Adenine
Guanine
- 2 CN rings (4N)
Guanine
- 2 CN rings (4N)
14
New cards
Pyrimidine
Cytosine
Uracil
Thymine
(Benzene C6H6)
- 1 CN ring (2N)
(Cut the pyramid)
Uracil
Thymine
(Benzene C6H6)
- 1 CN ring (2N)
(Cut the pyramid)
15
New cards
Purine Formation
(Adenine, Guanine)
spontaneous photochemical hydrolysis of cyanide
Adenine is 5 HCN's
spontaneous photochemical hydrolysis of cyanide
Adenine is 5 HCN's
16
New cards
System
Reduction of System
17
New cards
Ammonia
:NH3
18
New cards
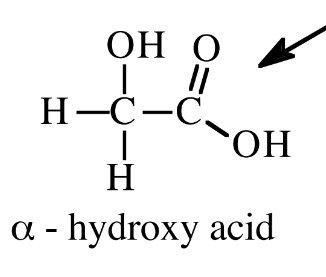
alpha hydroxy acid
19
New cards
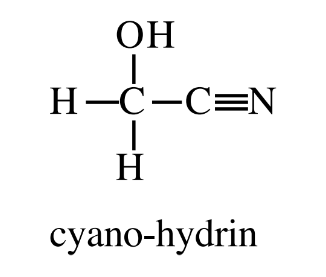
Cyano-hydrin
20
New cards
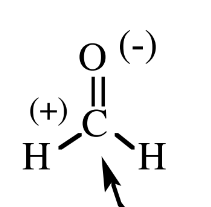
Formaldehyde
21
New cards
aldehyde
22
New cards
hydrocyanide
HCN
23
New cards
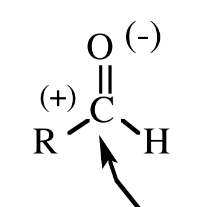
Aminoacid from striker (look like)
Could be Alanine or Glycine depending on the group
24
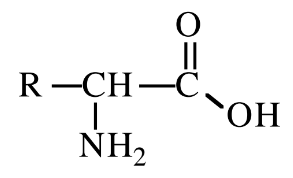
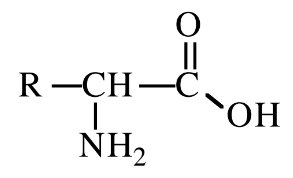
New cards
Alanine
amino acid with R group = CH3
25
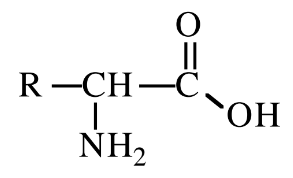
New cards
Glycine
Amino acid with R group = H
26
New cards
Adenine Structure
A purine (DNA base) made of 5 CNs
Note: guanine is an extra hydration step
Note: guanine is an extra hydration step
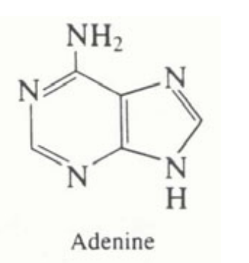
27
New cards
Methane
CH4
28
New cards
Pyrimidine synthesis
Harder to make than purine.
One theory is instead of Base + sugar makes base and sugar together.
One theory is instead of Base + sugar makes base and sugar together.
29
New cards
Chemical Polymerization
Connecting molecules together chemically
30
New cards
Thermal Polymerization
(unlikely)
Drying out and readding water.
150 -180C exposures
Drying out and readding water.
150 -180C exposures
31
New cards
Glycerol Nucleic Acid and Threose Nucleic Acid
Pros and Cons
Pros and Cons
GNA and TNA
- Simpler than RNA, but can be read by the same systems
- Decays faster (Unstable)
- Simpler than RNA, but can be read by the same systems
- Decays faster (Unstable)
32
New cards
Peptide Nucleic Acid
PNA
- simple and easy to make
- but not sugar backbone (v different)
- simple and easy to make
- but not sugar backbone (v different)
33
New cards
HCN polymer
- HCN is abundant and while spontaniously do it
- too abundant so it's hard to control
- too abundant so it's hard to control
34
New cards
Chondrules
Small inclusions in undiffernchaited metiories
35
New cards
Chondrites
Account for 84% of meteorites
undifferentiated rock
undifferentiated rock
36
New cards
Carbonaceous Chondrites
.5-5% carbon
2 - 10% H20
2 - 10% H20
37
New cards
Pallasite Meteorite
Find in 1951. A stony iron, core mantel boundary.
38
New cards
Why is a methane atmosphere not sustainable
Methane photolyzes in UV on a 100 Myr timescale
39
New cards
Ribozome Protein/RNA
Need RNA more. RNA made Ribosome
40
New cards
Strings of Nucleic Bases are hard to make
Poly A is good, rest much worse. If add isomers only really short chains can be made
41
New cards
Uranium 238
Lead 206
4.5 Ga
4.5 Ga
42
New cards
Uranium 235
Lead 207
0.7 Ga
0.7 Ga
43
New cards
Stable Lead isotopes
Lead 204
44
New cards
Rubidium 87
48.6 Ga
Strontium 87
Strontium 87
45
New cards
Reverse Gyrase
Hydrothermal supercoiling adaptation used in PCR
46
New cards
On Axis circulation
350-370C
24 Km^3/yr
Less and hotter
Near MOR
24 Km^3/yr
Less and hotter
Near MOR
47
New cards
Off Axis Circulation
150 avg C
560 km^3/yr
More and Cooler
Far from MOR
560 km^3/yr
More and Cooler
Far from MOR
48
New cards
Magnetite
Fe3O4
49
New cards
Iron Oxide
FeO
50
New cards
Fayalite
Fe2SiO4
Iron Olivine end member
Iron Olivine end member
51
New cards
Pyrrhotite
FeS
52
New cards
Pyrite
FeS2