R3.4.9 SN1 and SN2 mechanisms
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
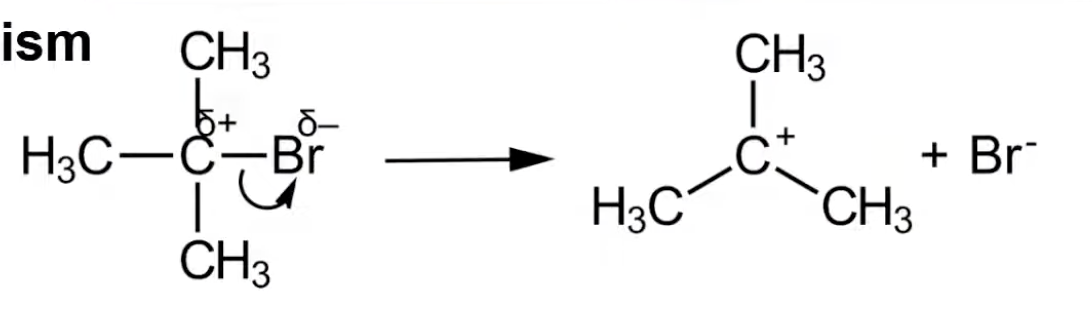
Two-step reaction: forms a carbocation intermediate.
Nucleophile bonds to the carbocation.
Common in tertiary halogenoalkanes.
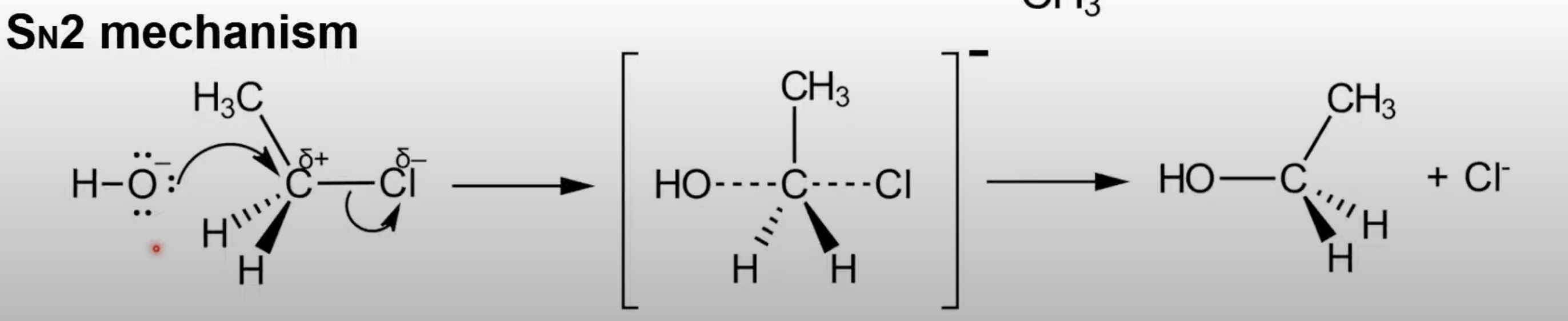
One-step reaction: nucleophile attacks opposite to leaving group.
Forms a transition state.
Common in primary halogenoalkanes.
C–X bond undergoes heterolytic fission.
Forms carbocation and halide ion.
Carbocation stabilized by inductive effect.
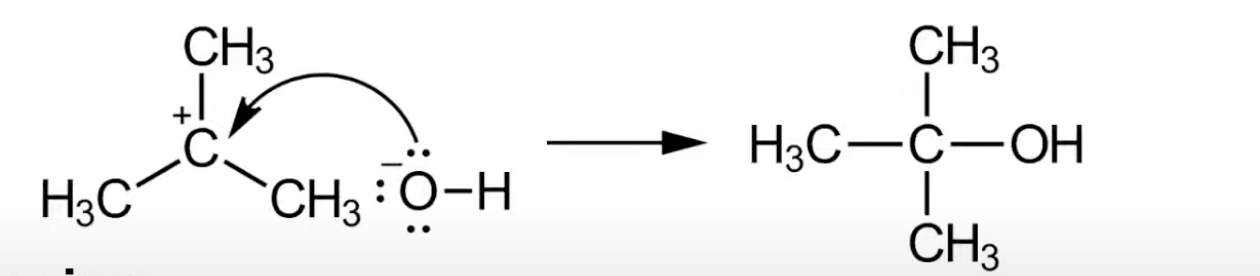
Nucleophile donates a lone pair to carbocation.
Forms new C–Nu bond. Leads to alcohol product.
Carbocation is trigonal planar.
Nucleophile attacks from either side.
Can lead to racemic mixtures.
Bulky alkyl groups prevent backside attack.
Promotes SN1 over SN2.
Seen in tertiary halogenoalkanes.
Backside attack by nucleophile.
Nucleophile and leaving group weakly bond in transition state.
Results in inversion of configuration.
Rate depends on halogenoalkane concentration.
Unimolecular rate-determining step.
First-order kinetics.
Rate depends on halogenoalkane and nucleophile.
Bimolecular reaction.
First-order in both reactants.
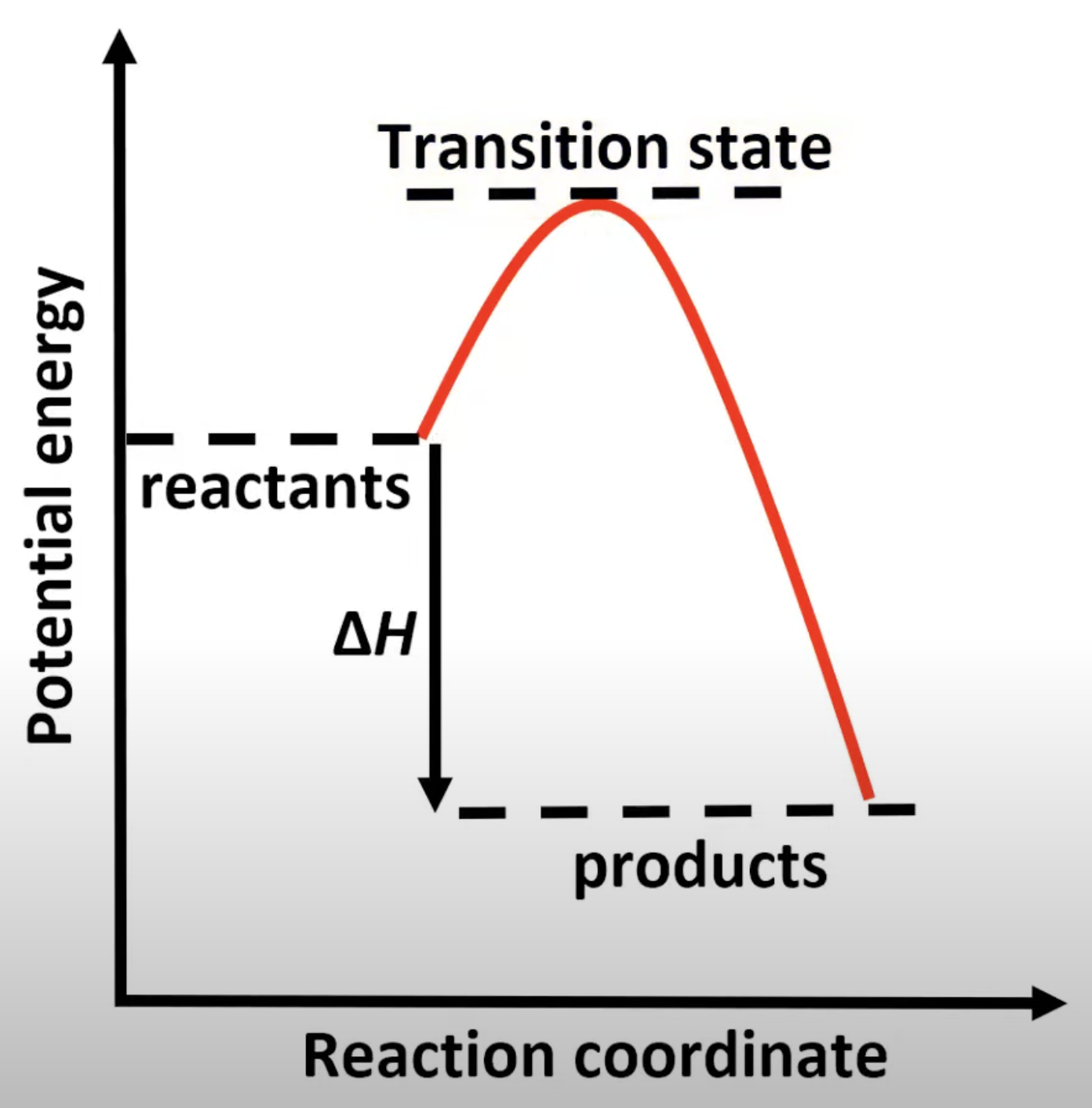
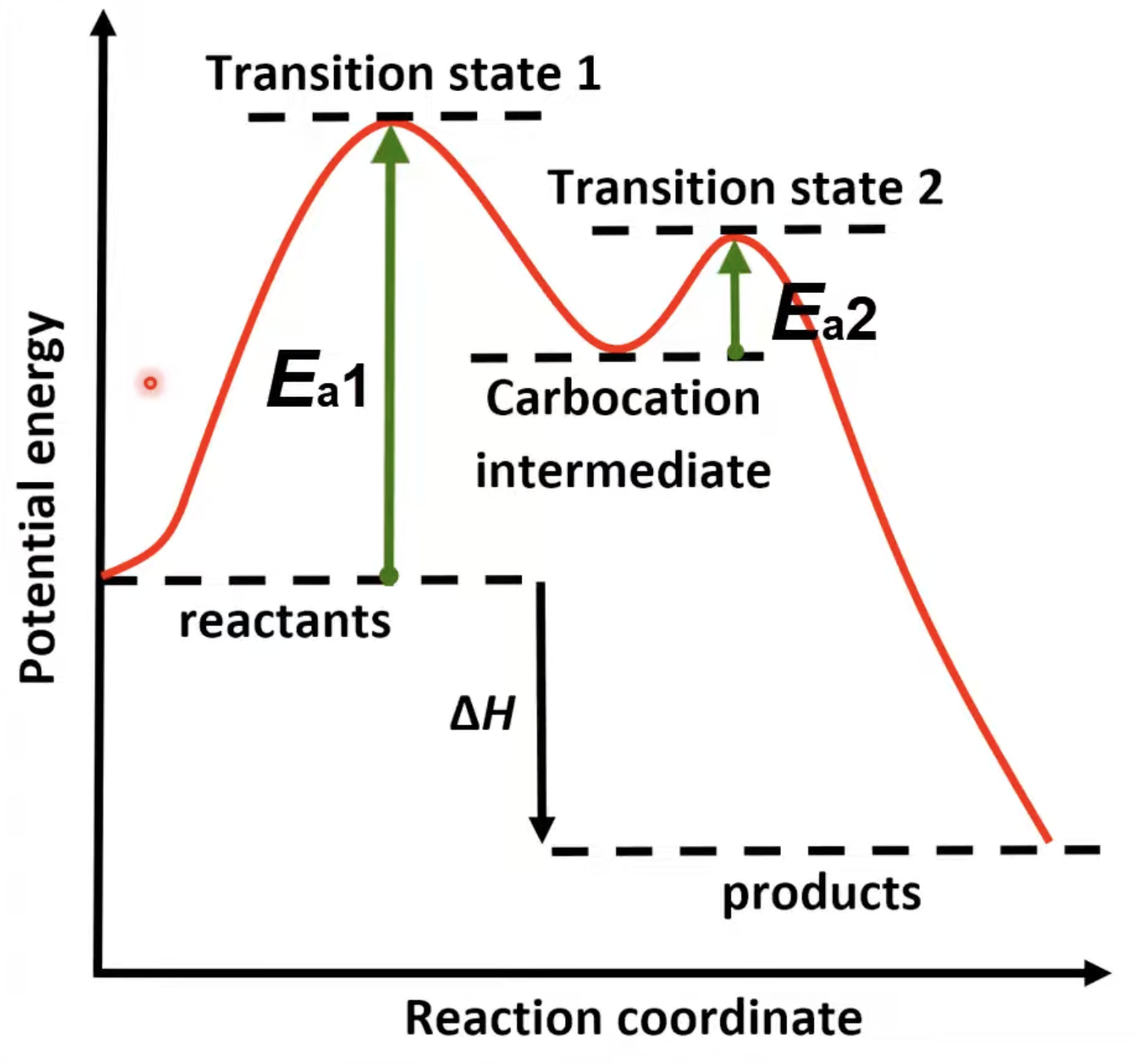
Two peaks for two steps.
First step has higher activation energy.
Carbocation intermediate forms.
Single transition state.
One activation energy peak.
Involves simultaneous bond breaking and forming.