Cell Bio. Learning Objectives - Exam One
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
1.1 Compare and contrast different cell types and their shared features and chemistry and list the basic ideas of the “cell theory”
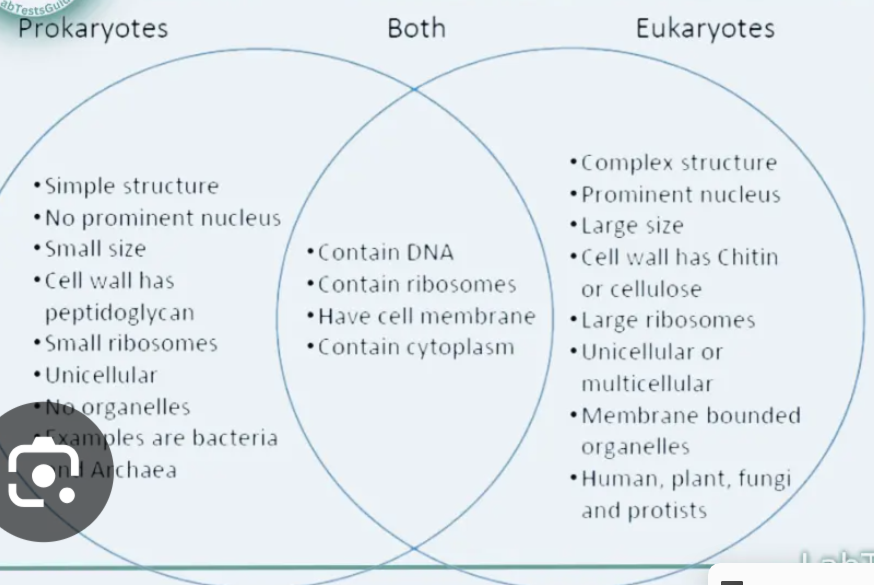
There are two different types of cells are prokaryotic cells lack a nucleus, few organelles vs eukaryotes have membrane bound organelles (organelles with their own membranes) and DNA contained in the nucleus. However both cell types are made out protein, carbohydrates, nucleic acid and lipids which are made out of carbon, hydrogen, oxygen and nitrogen. P and E and both have ribosomes and cell membrane
Cell Theory
All organisms are made out of cells one or more
All cells arise from pre-existing cells
Cells are the structural and functional unit of all living things
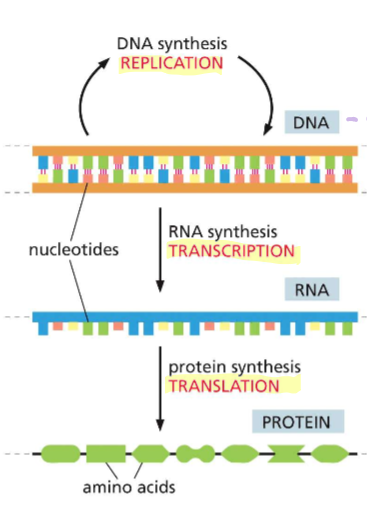
1.2 Explain the Central Dogma and how it relates to self-replication in living cells and differential form/function in cells
The Central Dogma is when goes genetic information flows from (replication) DNA - RNA (transcription) - Protein (translation) crucial for self-replication in living cells as it allows for the accurate copying of genetic information to produce new proteins necessary for cell division and function
Different function in the cell because different sequences of nucleotides will code for different proteins
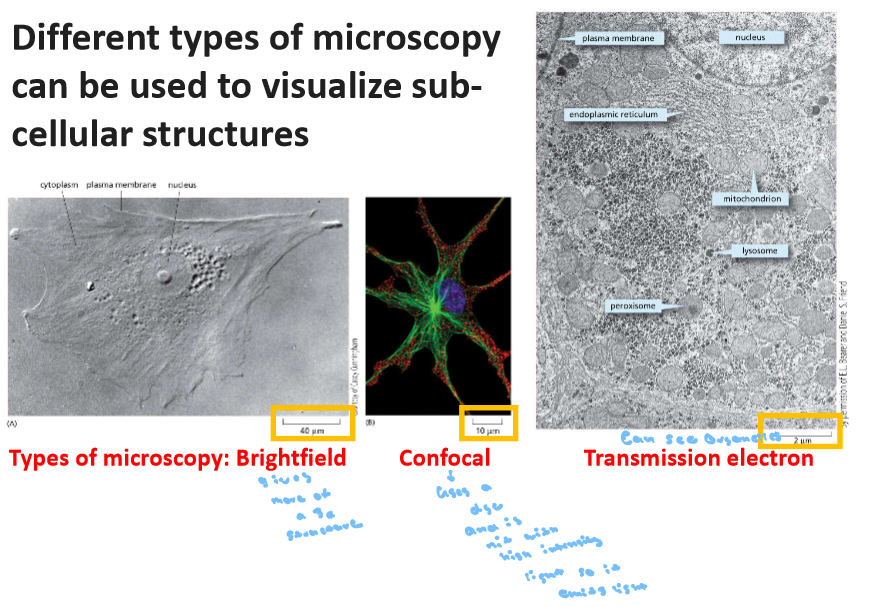
1.3 Compare and contrast some of the different ways in which cells can be visualize
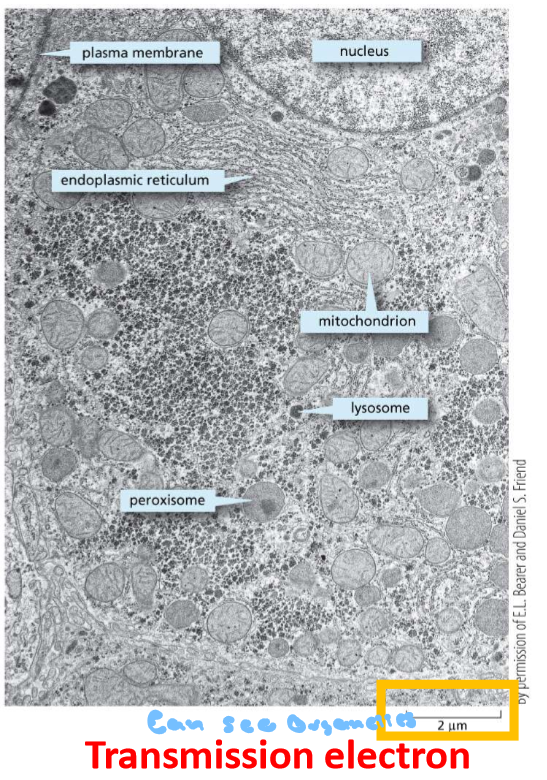
Transmission - Can see the organelles and in black and white
Transmission Scanning Electrons Microscopy - use electrons to super tiny as small as about 1 nm in biological specimens can see 3 to 20 n
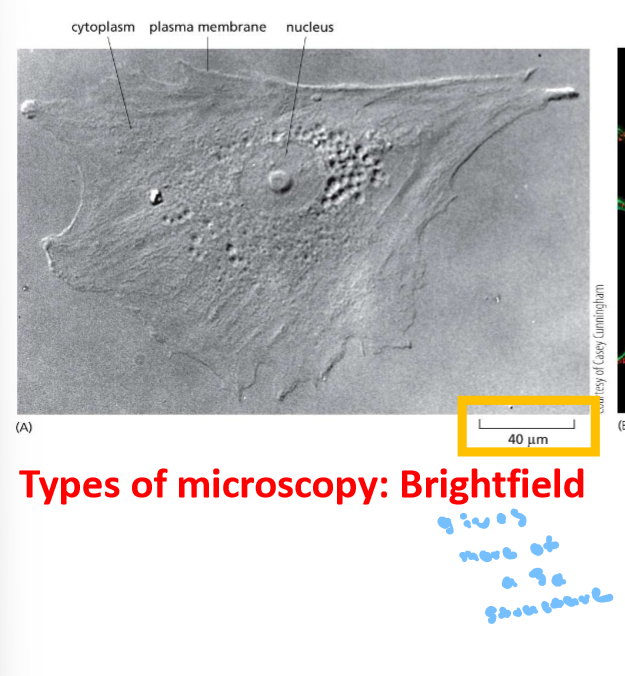
Light Microscopy - black and white and can have a stain also ind. cells and full cell structure and few organelles
Confocal Fluorescence Microscopy - Florence is used
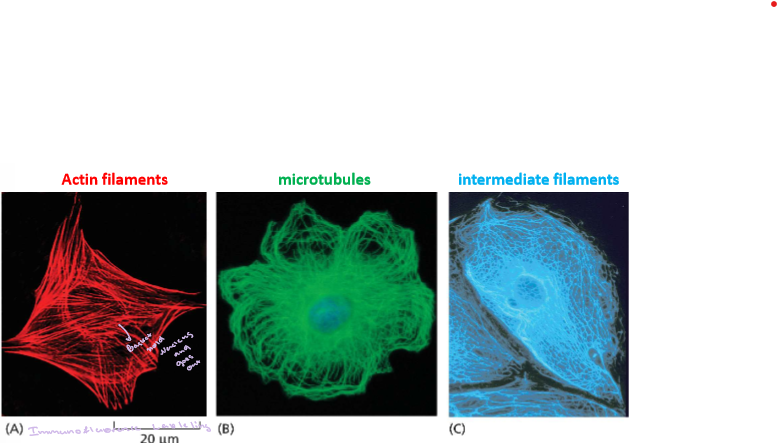
1.4 Explain the differences and similarities between prokaryotic and eukaryotic cell

1.5 Describe current ideas regarding the origin of mitochondria and chloroplasts as eukaryotic energy organelles
The current ideas regarding the origin of mitochondria and chloroplasts. Is long ago a bacterial ectosymbiont was engulfed by eukaryotic cells and the bacterial ect. becomes a symbiont evidence of widely accepted due to evidence like their double membrane structure, separate DNA, and similar size to bacteria
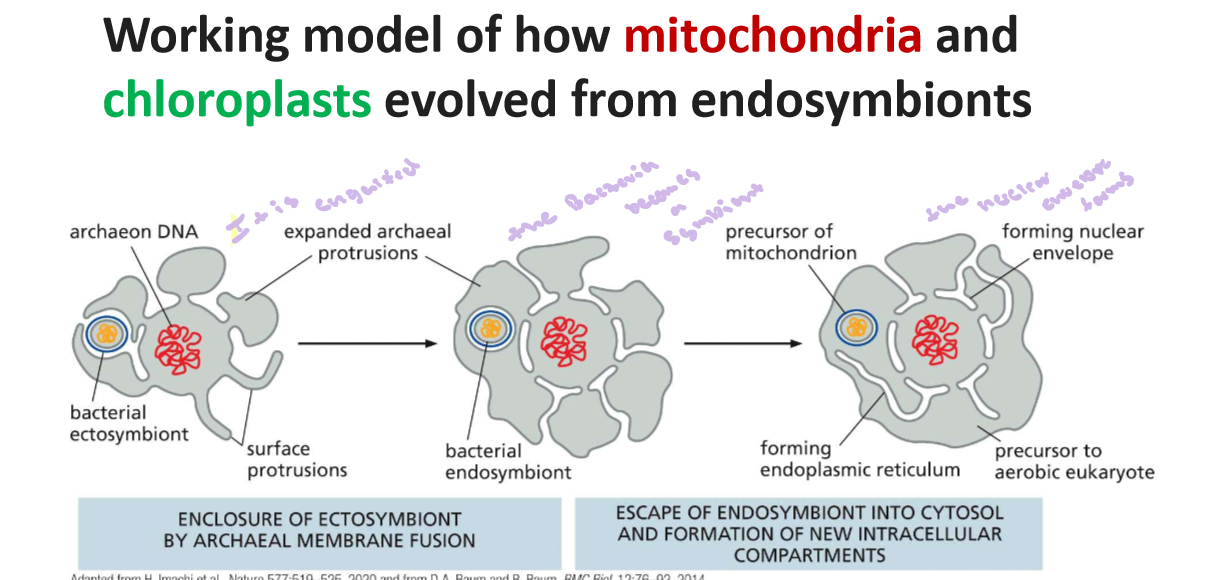
we all started form a prokaryotic cell and archaea when to the eukaryote and earlier verion of eurkayotes

1.6 List and understand the structure-function relationships in the different organelles and subcellular components of eukaryotic cells
Endoplasmic reticulum (ER) - since it so close to nucleus material exet out of there and has lots of cell that secrete large amounts of proteins
Golgi apparatus - modifies and packages molecules made in the ER to go outside of cell or somewhere in the cell
Lysozomes - intracellular digestion it releasing nutrients form ingested food particles back in the cytosol and breaks down unwanted molecules recycles them
Peroxisomes - uses hydrogen peroxide to inactive toxic molecules transport vesicles
Ribosomes - molecular machines that synthesize proteins in all living cells. They are made of RNA and protein, and are found in large numbers in cells
Transport Vesicles : movement of intracellular material
1.7 Understand why scientists use model organisms in research and explain some of the advantages-disadvantages of some examples of typical model organisms & viruses
put stuff from ala
E coli is often used as a prokaryotic model, particularly in molecular biology
Yeast (i.e. Brewer’s Yeast) cells are used as a model for simple eukaryotic cells
Nematodes (C. elegans) and fruit fly (Drosophila melanogaster) are common invertebrate models
Zebrafish (Danio rerio) and mouse (Mus musculus) are common vertebrate model systems
Homo sapiens studies in biomedical research and in genomics research have expanded our understanding of human development and disease….much more to learn!
Valuable tools include in vitro models (i.e. cell culture and organoid culture)
Bioinformatics and computational approaches aid comparisons across genomes
SARS-CoV-2 Genomic sequencing studies led to rapid development of mRNA-based vaccines against this Coronavirus
Current analysis highlights SARS-CoV-2 as a potential model to study eukaryotic cell infection mechanisms
1.8 Understand the basic relationships and the major domains in the Tree of Life
Bacteria - Prokaryotes such E.coli and yeast
Archaea - Prokaryotes
Archaea are often extremophiles capable of living in a broad range of habitats
Eukaryotes - Include Animals, Fungi, Plants
Bacteria and Archaea are primarily single-celled prokaryotes, while Eukarya includes more complex organisms like animals, plants, and fungi with eukaryotic cells containing a nucleus.
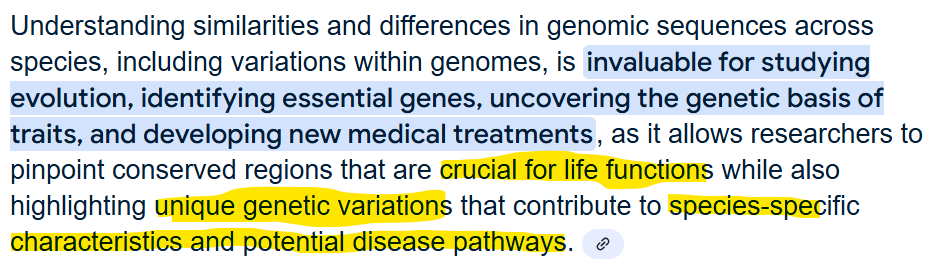
1.9 Explain the value in understanding the similarities and differences in genomic sequences as well as variation in genomes across species
Genome size varies across organisms
See Fig 1-43 (pg 38) for comparison
Homologous genes display conserved nucleotide sequences and sometimes similar functions
Not all genomic sequence codes for genes—some sequences and sequence regions are regulatory domains

2.1 Review the basic properties of atoms and bonds.
Covalent Bond - Sharing of Electrons
Ionic Bonds when - Transfer of Electrons
Atomic Number - Number of Protons
Atomic Mass - Protons + Neutrons
2.2 Distinguish between elements, atoms, ions, isotopes, and molecules.
Elemsents are pure subtance atoms smallest unit of elements whihc are made up of protons and neutrons and electrons. An ion is a chaged atom meaning if gained or lost elections istopes are a varietn of an element with the same nom of protons but diffl neutorns.
2.3 Recognize and understand the number of covalent bonds that can be formed by atoms of hydrogen, oxygen, nitrogen, and carbon and express how the electronic configuration of carbon, in particular, dictates the three-dimensional shape of organic molecules.
Hydrogen (H):
Can form one covalent bond because it has only one valence electron, needing one more to complete its outer shell.
Oxygen (O):
Can form two covalent bonds as it has six valence electrons, requiring two more to reach a stable octet.
Nitrogen (N):
Can form three covalent bonds due to its five valence electrons, needing three more to complete its outer shell.
Carbon (C):
Can form four covalent bonds because it has four valence electrons, allowing it to share four electrons with other atoms to achieve a stable octet.
2.4 Differentiate between covalent and ionic bonds in terms of their electronic configuration, strength and stability, and their prevalence and role in biological systems
Covalent: share electrons (negative charge cloud; dense between 2 positive charge nuclei). short, short bond length, very stable and require more energy to break.
connects monomer to form polymers. found in permanent place.
Ionic: electrons are donated by one atom to another. weaker than covalent but stronger than other non-covalent interactions, forms interactions between polymers.
Found in non-permanent place, very disruptive
--example: putting into water with salt, it will dissolve
Covalent bonds link together the subunits in a macromolecule and allow rotation of the atoms to induce flexibility. Weaker, noncovalent bonds form between different parts of the molecule. They ensure that the polymer adopts one particular conformation, determined by the linear sequence of monomers in the chain.
2.5 Express how the chemical and physical properties of methyl groups (−CH3), hydroxyl groups (−OH),
carboxyl groups (−COOH), phosphate groups (−PO32–), and amino groups (−NH2) influence the
behavior of molecules in which these groups typically occur
Methyl (-CH3) - nonpolary and hydrophobic, making molecules containing them less soluble in water likely to interact with other nonpolar molecules ( often found in fatty acid chains)
Hydroxyl groups (-OH) - polar and hydrophilic - help form hydorgen bonds - form h bond with water and polar molecules
Carboxyl Groups (-COOH) - polar, acidic can ionize to form negatively charged carboxylate ion - molecule hydrophilic nature and potential for forming ionic bonds
Phosphate Group (-PO32-) - highly polar, negatively charged - often involved with stable phosphodiester bonds - important for energy storage
Amino Group (-NH2) - polar,basic - can accept protons - making molecules containing them potentially positively charged and able to interact with other polar molecules through hydrogen bonds. impacting protein structure and fucntion
2.6 Four main groups of small molecules
Four main groups of small molecules are sugar, fatty acids, amino acid, nucleotides
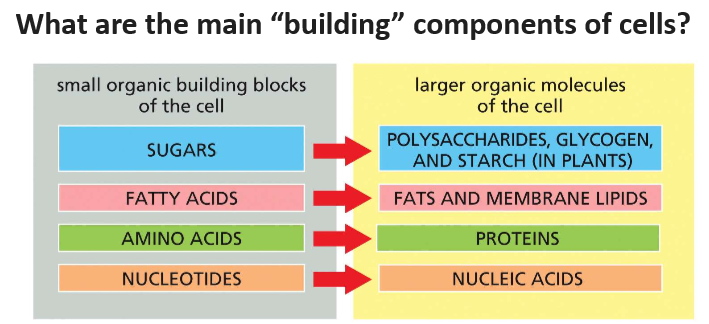
Sugars: These are the basic units of carbohydrates, which can be simple sugars like glucose or complex chains like starch.
Fatty acids: These are the building blocks of lipids, including fats and oils.
Amino acids: These are the subunits that make up proteins.
Nucleotides: These are the monomers that form nucleic acids like DNA and RNA.
2.7 Relate the different roles that sugars can play in the cell
Sugars, primarily in the form of glucose, play a crucial role in cells as a primary energy source, providing fuel for cellular processes through cellular respiration, but also act as signaling molecules, contributing to cell-to-cell recognition and regulating various cellular functions by attaching to proteins and lipids on the cell membrane; in plants, sugars also serve as a structural component in cell walls.
2.8 Summarize why the amphipathic nature of phospholipids is crucial for cell biology and predict how the saturation of fatty acid tails affects the fluidity of cell membranes
look at the ala
amphipathic nature of phospholipids is critical for cell biology because it allows them to spontaneously form lipid bilayers, the foundation of cell membranes, with their hydrophilic heads facing the aqueous environment and hydrophobic tails oriented inwards, effectively creating a barrier separating the cell interior from the extracellular fluid;. The saturation level of the fatty acid tails directly impacts membrane fluidity, where more unsaturated fatty acids lead to increased fluidity due to "kinks" in the tails preventing close packing, while saturated fatty acids with straight tails result in a more rigid membrane.
Key points:
Amphipathic nature:
Phospholipids have a hydrophilic phosphate head and hydrophobic fatty acid tails, enabling them to interact with both water and nonpolar molecules.
Bilayer formation:
This property allows phospholipids to spontaneously arrange themselves into a bilayer structure in aqueous environments, with the hydrophilic heads exposed to water and the hydrophobic tails facing each other.
Membrane fluidity:
The saturation level of fatty acid tails significantly impacts membrane fluidity.
Unsaturated fatty acids:
Presence of double bonds in unsaturated fatty acids creates "kinks" in the tails, preventing tight packing and increasing membrane fluidity.
Saturated fatty acids:
Saturated fatty acids with no double bonds can pack closely together, leading to a more rigid membrane.
The Lipid Bilayer - Molecular Biology of the Cell - NCBI
The tails are usually fatty acids, and they can differ in length (they normally contain between 14 and 24 carbon atoms). ... Diffe...
NCBI
Cell Membranes - The Cell - NCBI Bookshelf
The fundamental building blocks of all cell membranes are phospholipids, which are amphipathic molecules, consisting of two hydrop...
NCBI

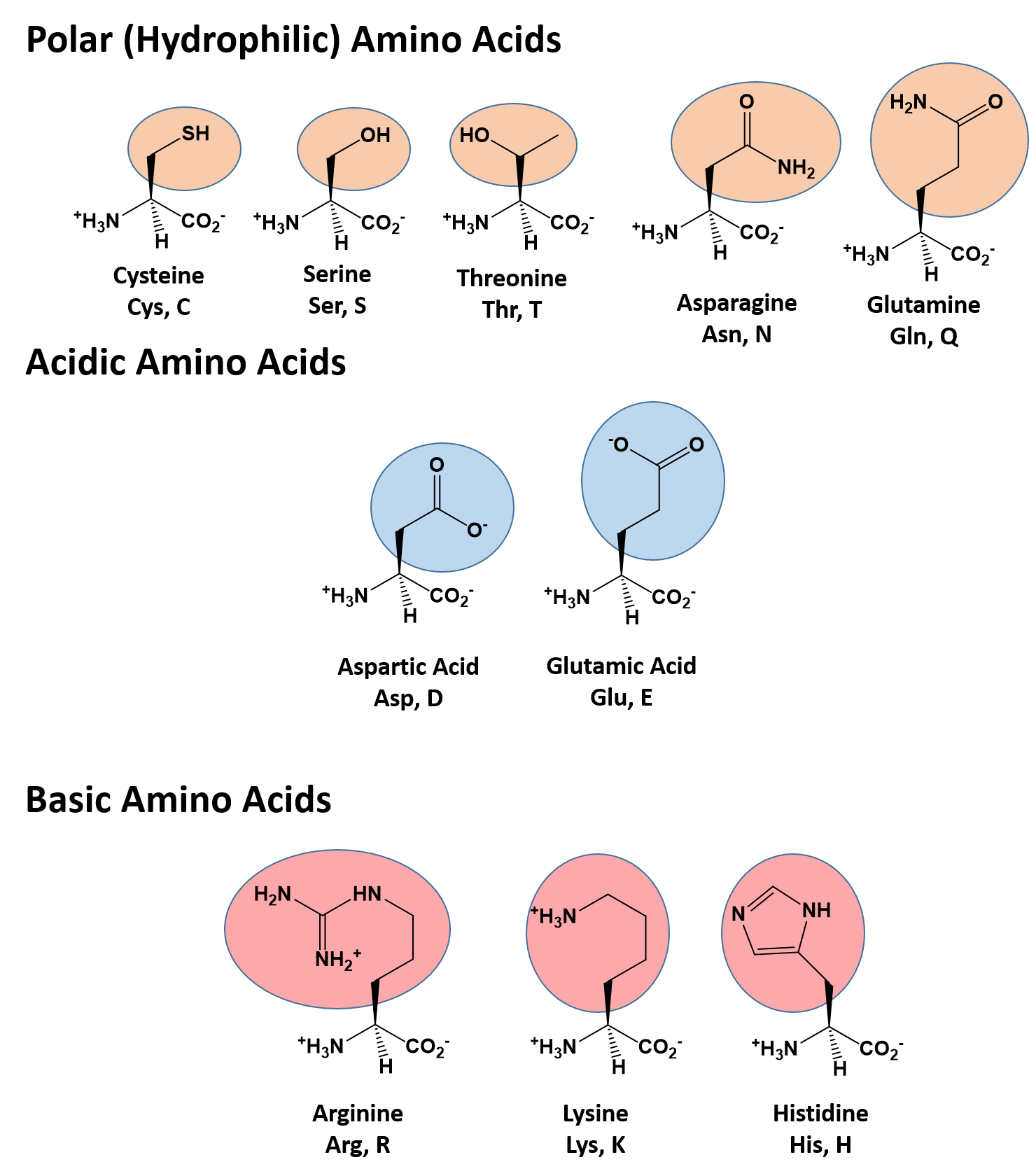
2.9 Identify the features that all amino acids have in common and understand how their structural
properties are linked to their function in proteins (review Panel 2-6)
a central carbon atom (alpha carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain called the "R group" which is what differentiates each amino acid and determines its unique chemical properties, ultimately influencing the protein's structure and function.
2.10 Express the differences between RNA and DNA and evaluate why these nucleic acids play different roles in cells
DNA primarily stores genetic information, while RNA acts as a functional intermediary, translating that genetic code into proteins by carrying the information to the ribosomes where protein synthesis occurs.
2.11 Understand how polymerization of monomers into polymers can yield macromolecules with diverse properties, structures, and functions
macromolecules with diverse properties, structures, and functions simply by varying the type of monomer used, the sequence of monomers within the chain, and the chemical bonds formed during polymerization, essentially acting like a molecular "building block" system where different combinations
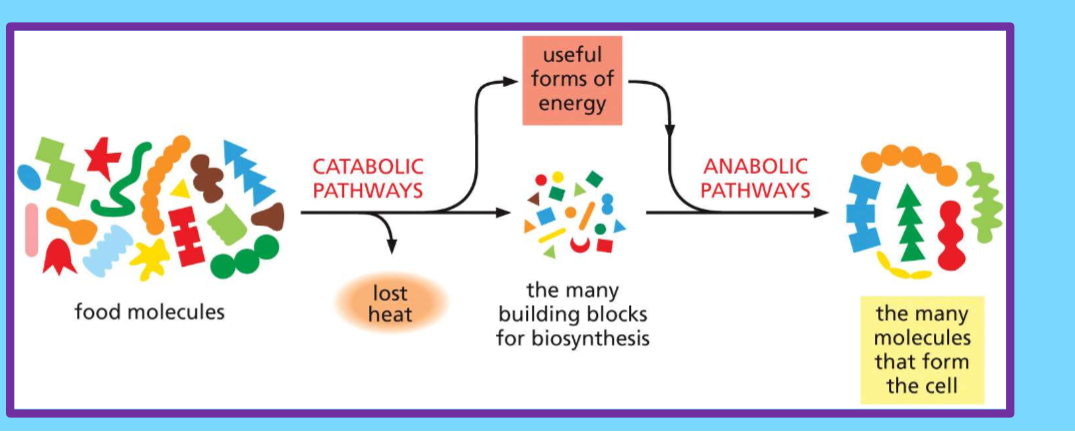
3.1 Relate anabolic, catabolic, and metabolic reactions to one another
Anabolic - build complex molecules, requires energy like ATP
Catabolic - breaks down molecules to create energy
anabolic and catabolic reactions are the two sides of the metabolic process, with one building up molecules while the other breaks them down, allowing for the necessary energy transfer within a cell.
Anabolic reactions:
Build larger molecules from smaller ones.
Require energy input (usually in the form of ATP).
Examples: protein synthesis (building proteins from amino acids), glycogen synthesis (building glycogen from glucose).
Catabolic reactions:
Break down complex molecules into simpler ones.
Release energy.
Examples: cellular respiration (breaking down glucose to produce ATP), digestion of food.
3.2 Summarize how the first and second laws of thermodynamics relate to living cells
The first law of thermodynamic is the energy is not lost gained in a closed system this relates for living cells cells can convert energy from one form to another and examples is the energy for sunlight is captured by the chlorophyll turns that energy in to sugar and that energy can be broken down into atp this shows how energy is not lost it is just changed
Cell takes in energy and uses it to gen. order within itself while heat is released therefore increasing entropy (disorder)
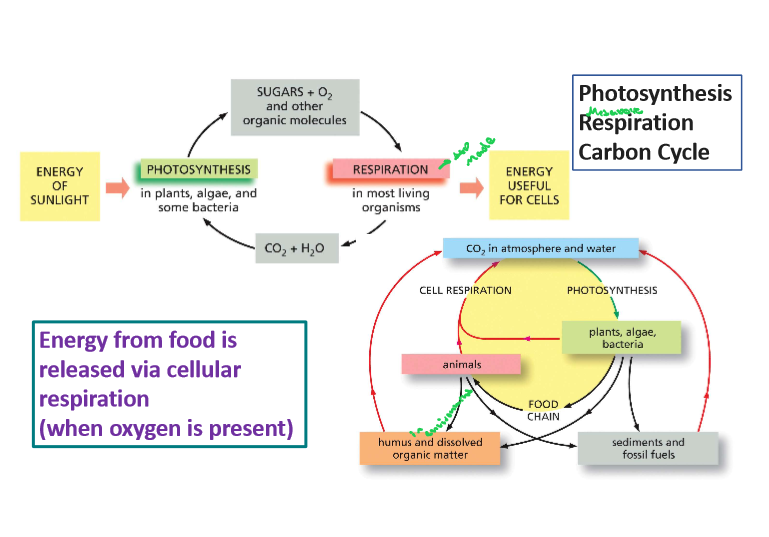
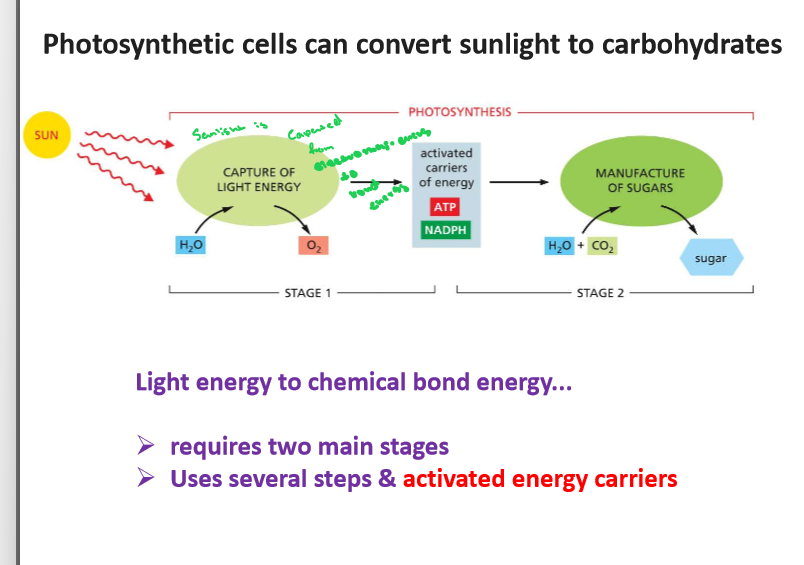
3.3 Contrast photosynthesis and cellular respiration (in general) and evaluate how the processes complement one another
Photosynthesis and cellular respiration are complementary biological processes where photosynthesis uses sunlight to convert carbon dioxide and water into glucose and oxygen, while cellular respiration breaks down glucose using oxygen to produce energy (ATP), carbon dioxide, and water; essentially, photosynthesis creates the fuel (glucose) that cellular respiration uses to generate energy, making them interdependent within the ecosystem.
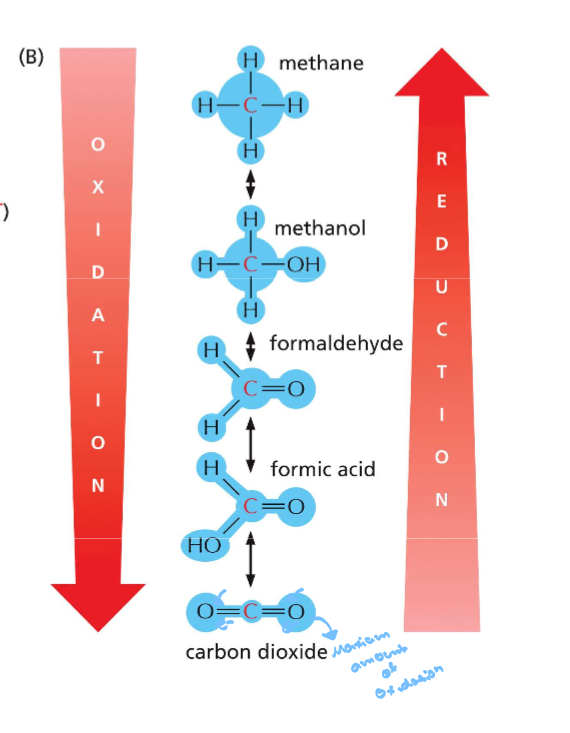
3.4 Differentiate between oxidation and reduction in terms of the movement of electrons, protons, and oxygen atoms
Oxidation is loss of electrons where their are more oxgens and less hydorgens is where and reduction is gain of electrons opp. for oxygens and less for hydrogens
oxidation has more bonds toward oxgen and reduced is gain bond to hydrogne


3.5 Describe how noncovalent interactions allow enzymes to interact with specific substrates


enzymes can change speed and rate don’t change the amount of heat

3.6 Explain why substrates for different enzymes can coexist in the same compartment
Substrates for different enzymes can coexist in the same compartment because each enzyme has a highly specific active site that only binds to its designated substrate, allowing for simultaneous reactions on different molecules within the same cellular space without interference, essentially acting like molecular "locks" that only fit their specific "keys" (substrates).
3.7 Illustrate how enzymes affect the equilibrium point of chemical reactions
Enzymes do not affect the equilibrium point of a chemical reaction; they only increase the rate at which the reaction reaches equilibrium by lowering the activation energy, meaning they speed up both the forward and reverse reactions equally, resulting in the same final equilibrium concentrations of reactants and products, just achieved much faster.
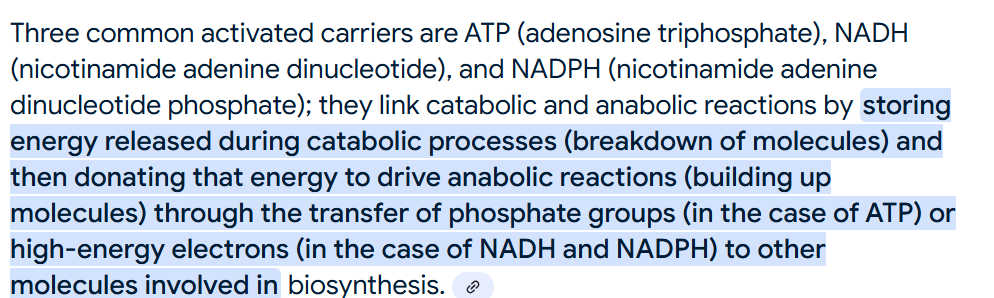
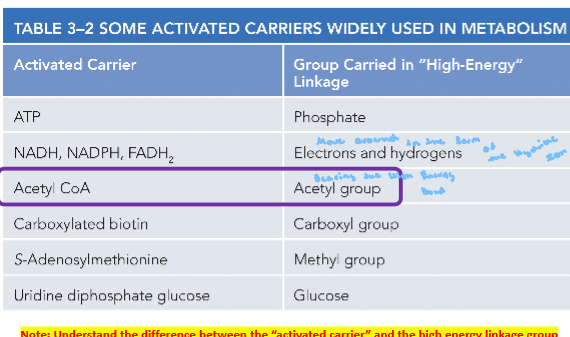
3.8 Be able to name 3 activated carriers and describe how activated carriers link catabolic and anabolic reactions
Ask in Tutoring

3.9 Relate how the energy of ATP hydrolysis can be harnessed to drive an energetically unfavorable condensation reaction (i.e. DNA synthesis)
Go Over

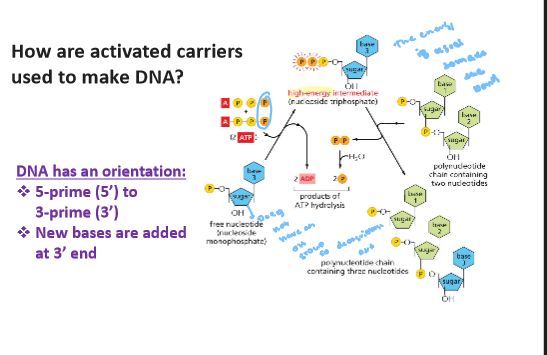
ATP hydroyis when atp donates the three phostphate group for a free nucleotide with is high - energy intermiediate so nucletides can be join toghter at 3 prime end wiht the energy the 2 phoste is relase water is realesed and a phosphate bonds are made

3.10 Evaluate the energetic favorability of condensation and hydrolysis reactions and state which type of reaction generally requires linkage to ATP hydrolysis in order to occur.
For water to bind as an h and a oh is easier to water to bind and hydrolysis to occur it is energetically favorable compared to condensation reaction when it has to release water
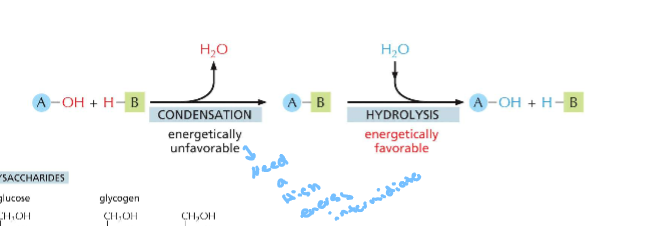
Condensation is energetically unfavorable while hydrolysis is favorable, condensation requires linkage to atp hydrolysis because you need evrgy to break the bonds to create water
