Chemistry - Buffers and pH + Solubility Product
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
buffer solution
a solution which minimizes pH changes when small amounts of acids or alkalis are added
acidic buffer
a mixture of a weak acid and its conjugate base (the salt of the weak acid)
What happens when acid is added to acidic buffer solution?
H⁺ ions combine with the conjugate base ions and the position of equilibrium shifts to the left and more acid is formed but due to the relatively high concentration of added base, the ratio of base to acid changes very little and the pH hardly changes
What happens when alkali is added to acidic buffer solution?
On addition of alkali to this buffer solution: OH⁻ ions combine with H⁺ ions to form water and the removal of H⁺ ions shifts the position of equilibrium to the right and more acid disassociates but due to the relatively high concentration of added base, the ratio of base to acid changes very little and the pH hardly changes
basic buffer
a mixture of a weak base and the salt of its conjugate acid
What happens when you add acid to basic buffer solution?
Base removes the added hydrogen ions and more salt is formed
What happens when you add alkali to basic buffer solution?
Base removes hydroxide ions to form water, the position of equilibrium shifts to the left and more base is formed
What are common organic buffer systems? (3)
Hydrogen-carbonate buffers, phosphate buffers and protein buffers
Describe hydrogen-carbonate buffers
Carbon dioxide combines with water in the blood to form hydrogen-carbonate ions which when the concentration of H⁺ ions increases in the blood, they combine with the hydrogen-carbonate ions which shifts the position of equilibrium slightly to the left and the concentration of H⁺ is reduced to keep the pH constant while still maintaining enough hydrogen-carbonate ions to allow the buffer to function
Describe phosphate buffer systems
In the cells, when H⁺ ions are added shifting the position of equilibrium to the left and the concentration of H^+ ions is reduced to keep the pH constant while still maintaining enough dihydrogen-phosphate ions to allow the buffer to function.
Describe protein buffer systems
Proteins can contain both the weakly basic -NH₂ group and the weakly acidic -COOH group and they can ionize as either acids or bases, depending on the surrounding pH, with either group serving as the buffer
Describe how Kₐ can be used to deduce the pH of a buffer
In the reaction, HA ↔ H⁺ + A⁻, Kₐ = [H⁺][A⁻]/[HA], where HA would be the acid, and A⁻ would represent the salt. Using this formula, [H₊] would be equal to Kₐ[HA]/[A⁻]. Resultantly, pH would be equal to pKₐ + log([A⁻]/[HA])
Describe how Kₐ would be used to determine the proportion of reagents necessary to change the pH of a buffer solution
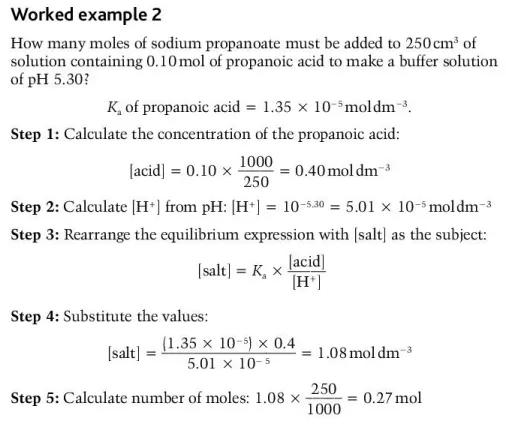
What are the steps for determining the pH of a buffer experimentally? (2)
Connect a glass pH electrode to a pH meter.
Dip the glass pH electrode into the buffer solution and read the meter.
solubility
the mass of a solute dissolve into a solvent at a fixed temperature and the ability of said solute to dissolve in the solvent
Kₛₚ (2)
an equilibrium constant that expresses the extent to which a sparingly soluble ionic compound can dissolve in a given solvent at a particular temperature
[A^+][B^-], where A and B are ions in solution.
Describe the principle of the equilibrium in Kₛₚ
When a sparingly soluble or insoluble salt is added to a solvent, an equilibrium is established between the ions in solution and the ions in solid in which ions move from the solid to solution at the same rate as they move from solution to solid
common ion effect
the instance in which the solubility of a solute in a solvent is reduced by the introduction of a solution of a compound which has an ion in common with the dissolved salt
How do we calculate the extent at which the common ion effect affects the solubility of an ion
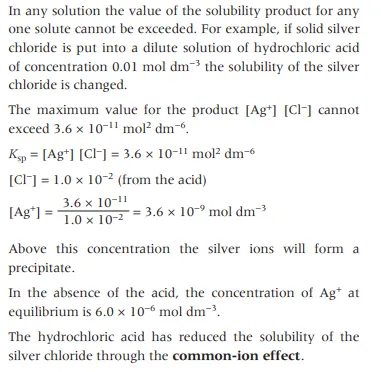
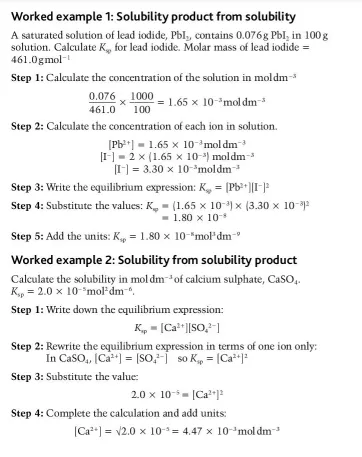
How do we determine the Kₛₚ of a substance experimentally?

How do we deduce the Kₛₚ of a substance using the solubility of the saturated solution?
How do we predict whether or not a precipitate will form when two solutions are mixed using Kₛₚ and the ionic product of the sal
If the ionic product is greater than Kₛₚ then a precipitate will form, if it is equal to Kₛₚ then the solution is saturated and if it is lower than Kₛₚ then the ions will dissolve without precipitation

How can we use solubility product data to choose the most suitable reagents for qualitative analysis?
Through choosing reagents that would form salts with low solubility products and would require less solution to form precipitates and derive results
Describe kidney stone formation
Kidney stones form as a result of an oversaturation of calcium salts in the urine which deposit as precipitates and build up. A higher concentration of calcium ions may also shift the position of equilibrium in favour of the precipitation of insoluble calcium salts, intensifying the effect of the oversaturation