Chromatography Techniques
0.0(0)
Card Sorting
1/87
There's no tags or description
Looks like no tags are added yet.
Last updated 6:33 PM on 9/20/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
1
New cards
Sketch a gas chromatograph
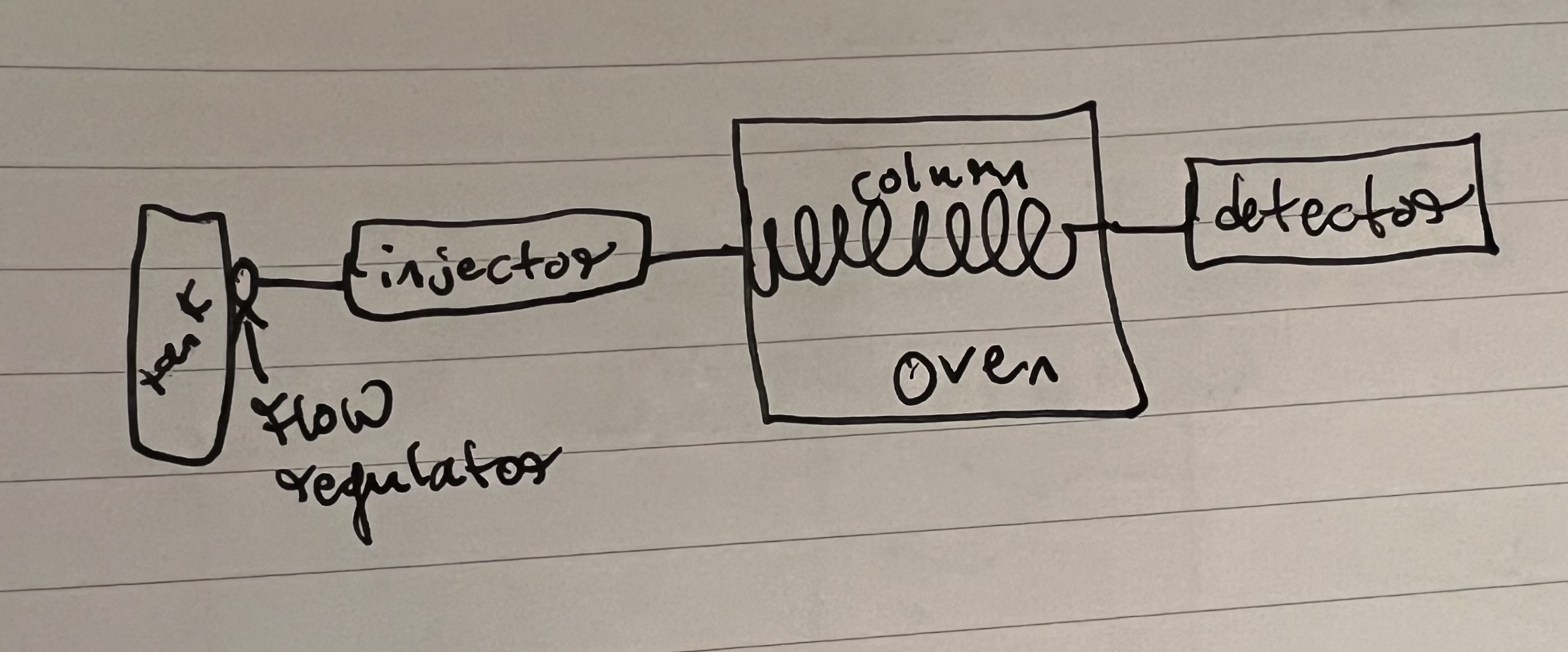
2
New cards
What are the major components of a GC system?
carrier gas, injector, column, oven, detector
3
New cards
Describe the characteristics of GC carrier gases
- mobile phase
- high in purity (99.99% or better)
- usually He (most common), H2, N2, or Ar (inert gases)
- high in purity (99.99% or better)
- usually He (most common), H2, N2, or Ar (inert gases)
4
New cards
What is the most common carrier gas for GC? What makes a carrier gas a good mobile phase?
He gas, inert
5
New cards
What can impurities cause in GC?
degradation of the column and cause false signals
6
New cards
How does an injector work?
sample is vaporized in the injector and injected into the gas stream, 1-10 microliters per injection, 30-50 degrees hotter than the highest oven temp., samples often split before reaching the column
7
New cards
What are the ONLY type of compounds GC can be used for?
volatile (b.p. less than 200, if can smell it can put it in GC)
8
New cards
What are the three types of columns used in GC?
packed with solid stationary phase, packed with inert solid support coated in liquid stationary phase, and open tubular
9
New cards
What are GC columns packed with solid stationary phases used for?
purifying gas, rare
10
New cards
Describe the properties of a GC column packed with an inert solid support coated in a liquid stationary phase
- 1-8mm in diameter
- made of glass or stainless steel
- 2-10m in length
- packing material 5-10 micrometers in diameter
- liquid stationary phase ADsorbed
- varying polarity (more polar = higher boiling point)
- MUST be thermally stable and non-volatile and not react with the analyte
- made of glass or stainless steel
- 2-10m in length
- packing material 5-10 micrometers in diameter
- liquid stationary phase ADsorbed
- varying polarity (more polar = higher boiling point)
- MUST be thermally stable and non-volatile and not react with the analyte
11
New cards
What percent of compounds can be analyzed using GC?
5-10%
12
New cards
Describe the characteristics of an open tubular column in GC
- no solid support
- stationary phase bonded directly to the interior surface of the capillary column
- columns made of fused silica
- 100-500 micrometer diameter
- 10-100 micrometer thickness
- 10-100m long
- different functional groups for different polarities
- more efficient separation
- stationary phase bonded directly to the interior surface of the capillary column
- columns made of fused silica
- 100-500 micrometer diameter
- 10-100 micrometer thickness
- 10-100m long
- different functional groups for different polarities
- more efficient separation
13
New cards
What material is a GC column with a solid stationary phase packed with?
charcoal, molecular sieves
14
New cards
What material is a GC column packed with an inert solid support coated in liquid stationary phase made of?
glass, stainless steel
15
New cards
What material is a GC open tubular column made of?
fused silica
16
New cards
List characteristics of an ideal detector
- low limit of detection
- wide linear response range (can detect a wide range of concentrations)
- uniform response to a variety of compounds
- simple calibration
- rapid response and recovery (
- wide linear response range (can detect a wide range of concentrations)
- uniform response to a variety of compounds
- simple calibration
- rapid response and recovery (
17
New cards
What does a thermal conductivity detector measure?
the change in resistivity of a heated wire in the gas stream due to the introduction of a compound
18
New cards
What are the pros and cons of a thermal conductivity detector (TCD)?
- Pros: universal, non-destructive
- Cons: low sensitivity (anything can make a signal), no structural info
- ?: requires a reference flow (mobile phase only)
- Cons: low sensitivity (anything can make a signal), no structural info
- ?: requires a reference flow (mobile phase only)
19
New cards
What is the most widely used GC detector?
flame ionization detector (FID)
20
New cards
How does GC-FID work?
analytes are burned in H2/air flame, analytes ionized and ion current detected
21
New cards
What are the pros and cons of GC-FID?
- Pros: more sensitive than TCD, almost universal
- Cons: destructive, no structural info
- Cons: destructive, no structural info
22
New cards
Which is more sensitive: FID or TCD?
FID
23
New cards
Which method is preferred: TCD or FID?
FID
24
New cards
How does an electron capture detector (ECD) work?
a radioactive source (63Ni) ionizes the sample, ions and e-'s generated, analytes containing electronegative atoms can "capture" the e-'s which reduces the current; can get a "dip" in the signal because the e-'s are sucked back up
25
New cards
What type of compounds is GC-ECD good for?
halogens, NO2, or P-containing analytes
26
New cards
What are the pros and cons of GC-ECD?
- pros: selective
- cons: destructive, no structural info
- cons: destructive, no structural info
27
New cards
How does a flame photometric detector work?
analytes are burned in flame, increased temp creates an excited state, photons are emitted from samples (detected by photo multiplier tube)
28
New cards
What are the pros and cons of a flame photometric detector?
- pros: selective
- cons: destructive, no structural info
- cons: destructive, no structural info
29
New cards
What compounds is a flame photometric detector selective for?
S and P
30
New cards
How does a mass spectrometer work?
the sample is fed into a "jet separator" to remove the majority of the carrier gas, analyte is ionized by e- impact, ions detected by m/z ratio
31
New cards
What are the pros and cons of a mass spectrometer detector?
- pros: gives structural info (qualitative data) on analytes, gives molecular weight (mass spectrum shows the fragments of a molecule with weights), mostly universal
- cons: EXPENSIVE ($30,000+), destructive
- cons: EXPENSIVE ($30,000+), destructive
32
New cards
Why is GC done at an elevated temperature?
to keep gases from condensing in the column
33
New cards
What are the two conditions for temperature in GC runs?
isothermal and gradient
34
New cards
What is an isothermal GC run?
the oven temperature remains constant throughout the run
35
New cards
Describe a temperature gradient in a GC run
the temperature ramps during a run (not constant), more volatile substances elute first (at low temp), less volatile elute after
36
New cards
What is the difference between liquid and gas chromatography (besides the liquid/gas)?
In LLC, samples interact with BOTH the mobile and stationary phases
37
New cards
How does an analyte interact with the mobile phase of LC?
the analyte dissolves in the liquid mobile phase
38
New cards
How does an analyte interact with the stationary phase of LC?
In LSC (liquid-solid chromatography), the analytes are absorbed by the solid. In LLC (liquid-liquid chromatography), the analytes dissolve in the liquid.
39
New cards
What are the two types of LC?
normal phase and reverse phase
40
New cards
What is normal phase LC?
- the mobile phase is LESS polar than the stationary phase
- less polar substances elute first
- can increase the polarity of the mobile phase to elute more polar analytes
- solvents MUST be miscible
- less polar substances elute first
- can increase the polarity of the mobile phase to elute more polar analytes
- solvents MUST be miscible
41
New cards
What is reverse phase LC?
- the mobile phase is MORE polar than the stationary phase
- more polar substances elute first
- gradient decreases the polarity of the mobile phase
- more polar substances elute first
- gradient decreases the polarity of the mobile phase
42
New cards
Describe the mobile phases of LC
- one or more solvents mixed together
- must be degassed (remove bubbles)
- composition of the solvent is an adjustable parameter (isocratic = solvent composition remains the same throughout the run, similar to isothermal function in GC)
- can have temperature gradients: linear ramp or gradient
- must be degassed (remove bubbles)
- composition of the solvent is an adjustable parameter (isocratic = solvent composition remains the same throughout the run, similar to isothermal function in GC)
- can have temperature gradients: linear ramp or gradient
43
New cards
What is the stationary phase of LC normally made of?
silica-based beads with liquid outside
44
New cards
What are the stationary and mobile phases of a normal phase LC made of?
- stationary: SiO2, Al2O3
- mobile: non-polar substance like hexane, pentane, benzene, etc.
- mobile: non-polar substance like hexane, pentane, benzene, etc.
45
New cards
What are the stationary and mobile phases of reverse phase LC made of?
- stationary: silica-based polymer, long chain alkanes
- mobile: polar substances like H2O, CH3OH, etc.
- mobile: polar substances like H2O, CH3OH, etc.
46
New cards
Describe the requirements of sample introduction in HPLC
- mobile phase supply system
- need a way to mix several solvents in varying ratios
- need a way to accurately control flow rate
- need a way to inject sample into flowing stream w/o losing pressure
- column must be able to withstand high pressure (up to 1000 psi for normal flow and up to 5000 psi for Ultra PLC)
- need a way to mix several solvents in varying ratios
- need a way to accurately control flow rate
- need a way to inject sample into flowing stream w/o losing pressure
- column must be able to withstand high pressure (up to 1000 psi for normal flow and up to 5000 psi for Ultra PLC)
47
New cards
What are HPLC columns made of? Why?
many made of stainless steel to withstand the pressure
48
New cards
What are the two types of pumps used in HPLC?
syringe pump and reciprocating pump
49
New cards
What is a syringe pump used for in HPLC?
lower pressure
50
New cards
What is a reciprocating pump used for in HPLC?
higher flow/pressure
51
New cards
Which pump is more commonly used in HPLC?
reciprocating
52
New cards
Which HPLC pump has better control?
reciprocating
53
New cards
How do injectors introduce samples into the stream in HPLC without losing pressure?
use a sample loop - inject a sample into the loop which may now seamlessly incorporate the sample into the mobile phase flow without interruption
54
New cards
What are the two types of columns used in HPLC?
- stainless steel columns: (1-4mm diameter), 5-150cm long, packed with 3-10 micrometer solid support
- nano-bore columns: can be much smaller (0.36mm diameter), can be made of fused silica
- nano-bore columns: can be much smaller (0.36mm diameter), can be made of fused silica
55
New cards
How does a refractive index detector work?
compares the refractive index of the solvent versus the solvent + the analyte
56
New cards
What are the pros and cons of a refractive index detector?
- pros: most universal HPLC detector, non-destructive
- cons: can't be used with solid gradient, no structural info
- cons: can't be used with solid gradient, no structural info
57
New cards
How does uv-visible absorption work?
analyzes the light absorption of analytes (analyte must absorb light while mobile phase doesn't, can't analyze more than one substance at a time)
58
New cards
What are the pros and cons of uv-vis?
- pros: sensitive, relatively universal, non-destructive
- cons: limited molecular info
- cons: limited molecular info
59
New cards
How does fluorescence work?
some molecules can absorb light at one wavelength and re-emit at another, fluorescence is proportional to concentration
60
New cards
What are the pros and cons of fluorescence?
- pros: very sensitive (zero background), non-destructive
- cons: very selective (most things don't fluoresce)
- cons: very selective (most things don't fluoresce)
61
New cards
How does fluorescence tagging work?
most compounds won't fluoresce but can be derivatized (tagged) to form fluorescent compounds
62
New cards
What are the two options for fluorescence tagging? How do you determine which to use?
- pre-column and post-column
- depends on the tag you want to use
- depends on the tag you want to use
63
New cards
Give an example of a fluorescence tag and tell when you would tag the compound (pre- or post-column)
- ninhydrin: reacts with primary amines so can tag amino acids, amine R-group does not end up in the product so ninhydrin must be used POST-column
- dansyl chloride: since R-group retained can do pre- OR post-column
- anthranilic acid: tag for carbs, can be used pre- OR post-column
- dansyl chloride: since R-group retained can do pre- OR post-column
- anthranilic acid: tag for carbs, can be used pre- OR post-column
64
New cards
Give pros and cons of using HPLC-MS
- pros: universal (better for polar molecules), compatible with electrospray ionization, lots of structural info
- cons: destructive
- cons: destructive
65
New cards
What is ion exchange LC?
separation depends on the charge density of the analytes: small compact ions bind more tightly then larger more diffuse ions, dications bind more strongly than mono-cations
66
New cards
Describe the stationary phase of ion exchange LC?
polymer with either positively or negatively charged side groups, substituted polystyrenes often used
67
New cards
What is size exclusion chromatography?
the stationary phase is a cross-linked polymer with very large uniform pores, smaller molecules get trapped in the pores whereas large ones pass through, retention inversely proportional to molar mass
68
New cards
What kinds of molecules is size exclusion chromatography used for?
proteins, polymers
69
New cards
How is qualitative analysis performed?
compare the retention time of the analyte to those of known standards under the same conditions
- requires pure reference compounds
- more than one compound can have the same retention time
- NOT best for true qual., but MS has improved it dramatically
- requires pure reference compounds
- more than one compound can have the same retention time
- NOT best for true qual., but MS has improved it dramatically
70
New cards
How is quantitative analysis performed?
compare peak heights or areas
71
New cards
What are the two types of analysis used?
calibration curves and standard addition
72
New cards
What is a calibration curve?
- creates standards of known concentration
- run on instrument under known conditions
- use peak height or area to create curve
- run unknown under same conditions and interpolate
- run on instrument under known conditions
- use peak height or area to create curve
- run unknown under same conditions and interpolate
73
New cards
Name some concerns in analysis
- linearity of Beer's Law
- variability of injection volume
- variability of injection volume
74
New cards
What approaches can be taken to address concerns in analysis?
- dilutions: can always dilute, can't always concentrate
- internal standards: add compound with similar structure to analyte to each standard and unknown in known concentrations, ratio of peak areas or heights eliminates sample to sample variation in injection volume
- internal standards: add compound with similar structure to analyte to each standard and unknown in known concentrations, ratio of peak areas or heights eliminates sample to sample variation in injection volume
75
New cards
Sketch a liquid chromatography setup
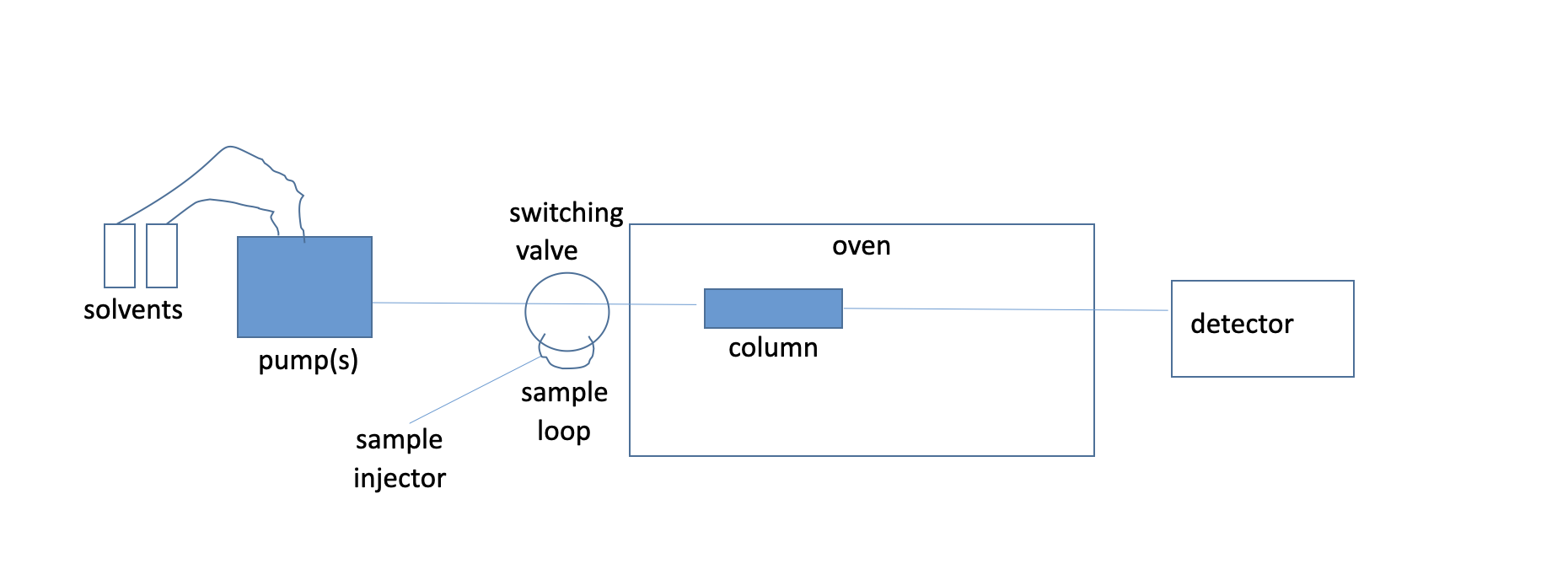
76
New cards
Sketch capillary electrophoresis
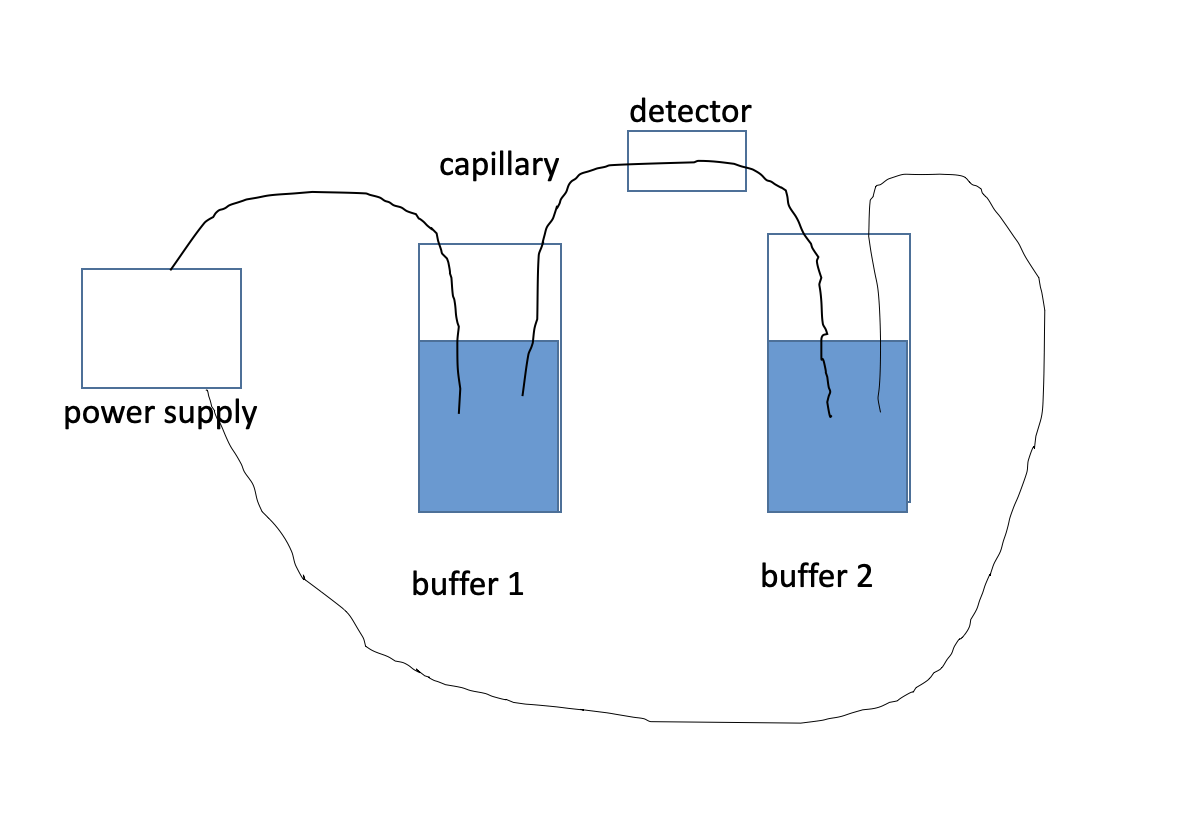
77
New cards
What are the two types of electrophoresis?
gel and capillary
78
New cards
How does gel electrophoresis work?
The sample is put in an x-linked polymer, a voltage is applied, and anions traveled down toward the anode.
- only works for charged species
- separation based on charge density (size)
- separation proportional to applied voltage, but resistive heating limits voltage
- only works for charged species
- separation based on charge density (size)
- separation proportional to applied voltage, but resistive heating limits voltage
79
New cards
What is gel electrophoresis good for separating?
DNA and proteins
80
New cards
What voltage can be applied in gel electrophoresis?
less than 500 volts
81
New cards
How does capillary electrophoresis work?
buffer solution instead of gel, small sample volumes, higher voltages (leads to VERY efficient separations, millions of theoretical plates), doesn't work for neutrals, many detectors available
82
New cards
What voltages can capillary electrophoresis be used for?
High voltages, 10s of kilovolts
83
New cards
What detectors are available for use with capillary electrophoresis?
uv/vis, fluorescence, MS
84
New cards
What are the two types of flow in capillary electrophoresis?
electrophoretic flow (ep) and electroosmotic flow (eo)
85
New cards
What is electrophoretic flow?
cations attracted to cathode, anions attracted to anode
86
New cards
What is electroosmotic flow?
silica capillary has anionic side groups above pH 1-2: cations in buffer attracted to wall of capillary forming an electric double layer, next layer rich in cations and attracted to cathode, results in net buffer flow towards cathode
87
New cards
What is separation based on in capillary electrophoresis?
combination of ep and eo flows results in separation of anions from cations (and neutrals), separation within charge classes is by charge density
88
New cards
What are the mobile and stationary phases of capillary electrophoresis?
stationary: NONE!
mobile: buffer
mobile: buffer