Reaction pathways
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
Substitute reactions
Alkane —> (Uv light + 2X) Haloalkane +HX
Haloalkane —> Amine/alcohol + HX
Addition Reactions
Hydrogenation: Alkene —> (Catalyst Pd/Pt/Ni) Alkane
Halogenation: Alkene —> (X+X) Haloalkane
Hydrohalogenation: Alkene —> (H+X) Haloalkane
Hydration: Alkene —> (H3PO4/high temp) Alcohol
Oxidation Reaction
Carboxylic acids - high temps
Aldehydes - low temps
Primary alcohol —> (Oxidising agent, Acid catalyst, High/low temp) Carboxylic acid/aldehyde
Condensation Reactions
Esterification: Carboxylic acid + alcohol —>(Catalyst H2SO4 + Heat) Ester + H2O
Synthesis of Lipids: Glycerol + 3x fatty acid —> (Enzyme) Triglyceride + 3H2O
Synthesis of Proteins: Amino + Amino —> Dipeptide +H2O
Synthesis of carbohydrates: monosaccharide + monosaccharide —> Disaccharide + H2O
Hydrolysis reactions
Esters: Ester + H2O —> (NaOH) Carboxylic acid + alcohol
Lipids: Triglyceride + 3H2O —> (NaOH) glycerol + 3 fatty acids
Proteins: Dipeptide + H2O —> (HCl + heat) amino + amino
Carbohydrates: Disaccharide + H2O —> (Enzyme) monosaccharide + monosaccharide
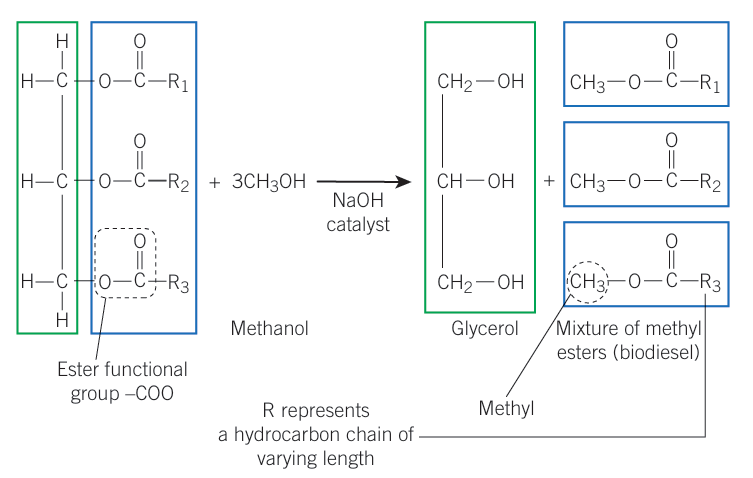
Transesterification of Biodiesel
Ester functional Group + 3x Methanol —> (NaOH) Glycerol + Mixture of methyl esters
Percentage Yield Formula
Actual yield/Theoretical yield x 100
Multistep: Multiple all percentages/100 X 100
Atom economy Formula
how many reactant atoms end up in the desired product
Single step: MM of desired product/ MM of all products x 100
Multistep: MM of desired product/ MM of all reactants x 100