Why is there temp change in chemical reactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
exothermic reaction
a chemical reaction that releases energy to its surroundings
example of exothermic reaction
Combustion
Oxidation
Neutralisation
Everyday examples of exothermic reactions
Hand warmers
Endothermic reactions
Takes in energy from the surroundings so the temp of the surroundings decreases
Examples of endothermic reaction
Thermal decomposition
the reaction between citric acid and sodium hydrogencarbonate
Everyday example of endothermic reaction
Sports injury packs
activation energy
the minimum amount of energy required to start a chemical reaction
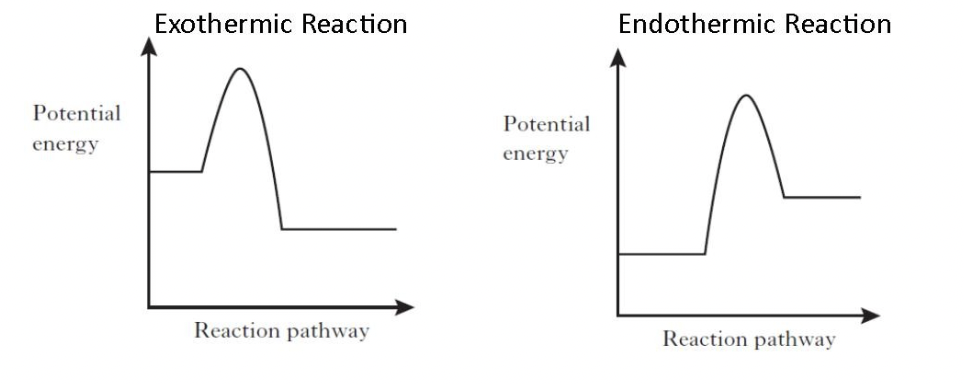
label both diagrams
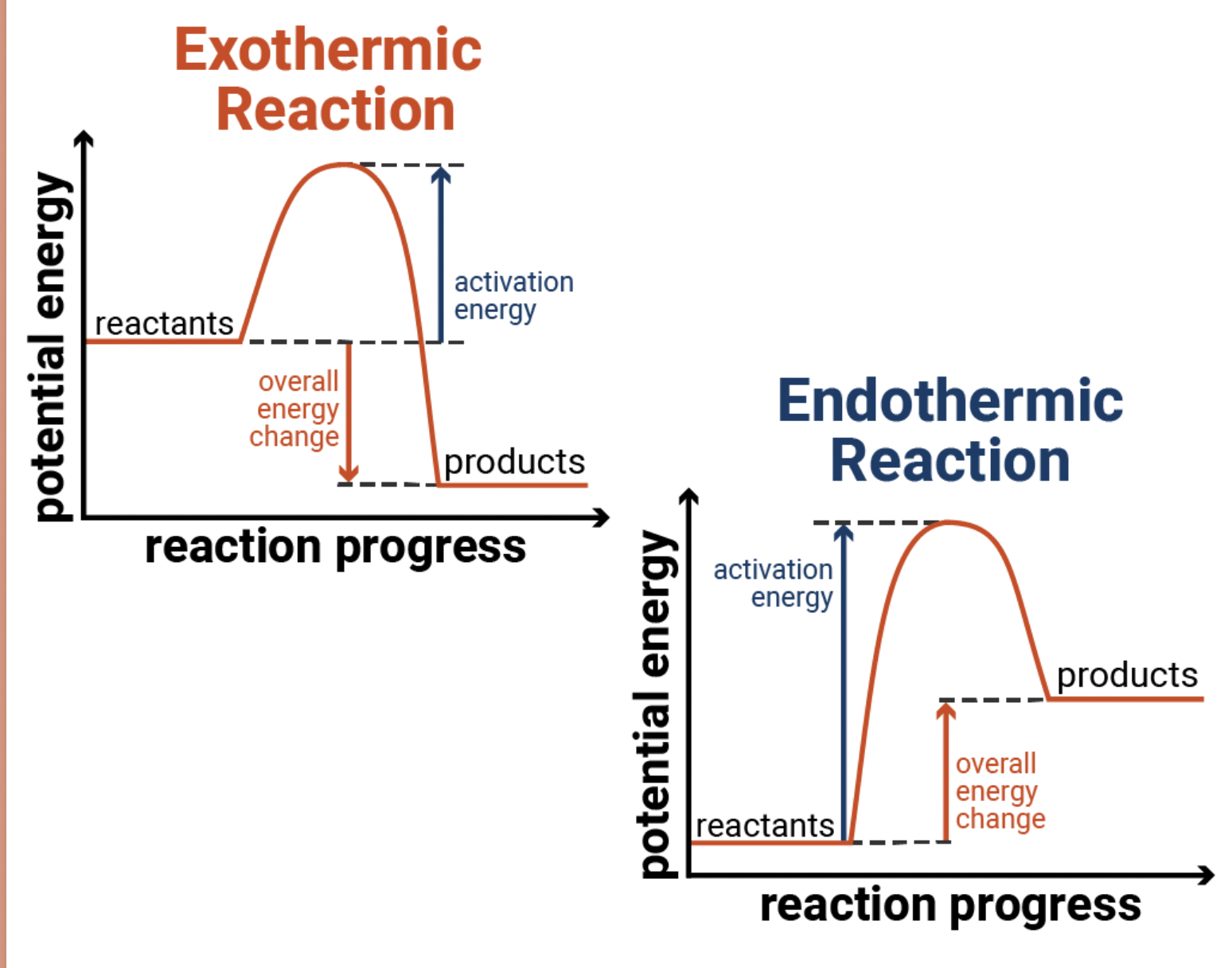
Practical for exothermjc or endothermic reaction
-take measurements of temperature before reaction with thermometer
-take measurements of temperatures at maximum/minimum point
-increase=exothermic
-decrease=endothermic
calculating energy change
energy in-energy out
negative change=exo
positive=endo