PTCB Prt 2- Federal Requirements
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
What are Hazardous Materials?
as any chemical or drug that poses potential harm to the person preparing or coming in contact with it.
What is OSHA?
Occupational Safety and Health Administration (OSHA)- is a federal agency within the US Department of Labor. Its primary mission is to ensure safe and healthy working conditions for all employees.
What is a Safety Data Sheet (SDS)?
is a document made by the manufacturer that provides detailed information about the hazards of a chemical product and guidance on safe handling, storage, and disposal.
When near or in contact with any hazardous material personnel must wear…?
personal protective equipment as outlined by the SDS (PPE)
Hazardous materials should be stored…?
Hazardous drug waste should be stored…?
Hazardous materials should be stored separately from non-hazardous materials in a negative-pressure room
Hazardous drug waste should be stored a leakproof container that is labeled as “hazardous drug waste in a negative pressure room.
What information is Required on Prescription Medication Labels?
1) name, address & phone # of pharmacy
2) Date of filling
3) name & address of patient
4) name of prescriber
5) Rx directions
6) Rx #
7) Drug name & strength
8) For CS: Warning label stating "Controlled substance dangerous unless used as directed"
Adulteration vs Misbranding
Adulteration- refers to the purity, quality, or strength of the drug or device being contaminated or inappropriate
Misbranding- refers to misleading labeling or a label lacking required information
OTC drugs must be labeled with:
active ingredients
amount of active ingredients in each dosage unit
inactive ingredients
the purpose of the product
specific warnings including when to contact the doctor,
possible side effects
directions for use
Lot Number
Pregnancy or breastfeeding warnings
"Questions? Call"
What are the different ways that prescriptions may be sent to the pharmacy?
by written hard copy, electronic transmission, facsimile transmission, verbal telephone order or verbal voicemail telephone message.
For non-CS prescriptions, how many refills may be transferred between pharmacies?
Only 1 refill may be transferred
What is the max number of refills a non-CS prescription can have?
A non-CS prescription can have 11 refills or less
What fields are required to be filled out on a prescription form? For CSs?
1) Patient name
2) Patient DOB
3) Date of issue
4) Drug Name
5) Drug Strength
6) Quantity
7) Directions
8) Number of Refills
9) Clinician name
10) Clinician signature
+ 11) for controls: Clinician DEA number is required
The Pharmacist in Charge (PIC) must notify the Board of any Theft of medications within how many days of the robbery?
The Board must be notified within 1 day
If there is a theft of CS, the DEA (Drug Enforcement Administration) must be informed within how many days?
DEA must be informed within 1 day
What DEA form must be filled out if there has been a theft of CS?
DEA Form 106
A copy of DEA Form 106 must be kept on file at the pharmacy for at least…?
2 years
The Electronic Controlled Substance Ordering System (CSOS) is the electronic equivalent of….?
The CSOS allows the purchaser to purchase...?
DEA Form 222
Sched I-II drugs. You can also use this system to order III-V, but it is not required
A Pharmacy needs to register with the DEA to…?
be able to order, process and dispense controlled substances
Reviewing medication orders allows Pharm Techs to…?
Are Pharm Techs involved in ordering C-IIs?
check that owed medications are on the order and that needed CIII-IV are ordered as well
No
Although each wholesaler has different policies on medication returns, if a pharmacist needs to return medications, what will happen?
The pharmacist will request a return
If the return request is approved, the wholesaler will send an authorization form either by mail or electronically. A return authorization will require a signature of a pharmacy employee and will provide details of how to package the return.
During the next medication delivery, the delivery driver will pick up the tote containing the return
If an investigational drug is considered to be a controlled substance AND the drug has been lost or stolen, then….?
The pharmacy must report the loss or theft to the DEA via DEA Form 106
Investigational or clinical trial drugs should be secured and stored in…?
Can all pahramcy staff work with the Investigational drugs?
in a separate room or separate storage area in the pharmacy.
No, only the pharmacy staff members that are authorized to work with the investigational drugs should have access.
When the products are shipped to the pharmacy, they should be clearly labeled as …?
The immediate container that holds the investigational drugs must contain the statement…?
as investigational drug products
“Caution: New Drug - Limited by federal law to investigational use.”
The following information is required to be on the pharmacy label for investigational drugs in addition to standard labeling requirements:
Name of the investigational drug product, unless blinded
Product strength or concentration, unless blinded
Quantity
Lot number, container number, or kit number
Expiration date or period of use
Manufacturer/sponsor name and address
Clinical research protocol number
What is a Drug Take Back Program?
a program that allows patients to return any expired or unused medications to an authorized medication collection site for proper and safe disposal.
What types of medications are usually not accepted at a Drug Take-Back Site?
are illicit drugs, inhalers and needles/sharps
Can C-II prescriptions be refilled?
No they cannot be refilled
However, they can be partially filled.
If a C-II is partially filled, the remainder must be filled within…?
For patients in long-term care facilities, or those who are terminally ill, the law allows C-II prescriptions to be…?
within 72hrs. If the remainder cannot be refilled, the remaining quantity is voided
be partially filled an unlimited number of times for up to 60 days after the date that the prescription is written.
How many times can a C-III or C-IV prescription be refilled?
Is there a limit to the number of times that a C-III or C-IV can be partially filled?
Up to 5 times in 6 months. CS prescriptions expire 6 months after they are written
There is no limit to the number of times that a schedule III or IV prescription may be partially filled.
Does federal law have any restrictions on the number of times that a C-V can be refilled?
There are no federal restrictions on the number of times that C-Vs can be refilled although many state laws set stricter requirements.
What must a perscriber do if they want to obtain a CS for office use?
Perscribers cannot write a prescription for office use Controlled Substances
Perscribers must use DEA Form 222 or the Controlled Substance Ordering System (CSOS) to place an order for C-IIs for office use
For C-III to C-V order must be placed via a valid order form from the. wholesaler
Can C-IIs be transferred between pharmacies?
They CANNOT be transferred between pharmacies
Can C-IIIs to C-IVs be transferred between pharmacies?
Can C-Vs be transferred between pharmacies?
They may be transferred between pharmacies only ONE time. Chain pharmacies that share a common database can transfer prescriptions an unlimited number of times.
Schedule V’s may be refilled without limits
Do Non-controlled substances have any restrictions on prescription transfers?
Non controlled prescriptions do not have any restrictions on prescription transfers and can be transferred between pharmacies and unlimited number of times.
What is a Material Safety Data Sheet (MSDS) ?
MSDS contain information on chemicals, their identifying characteristics, flammability, volatility, storage requirements, exposure prevention and what to do in case of a spill.
Every pharmacy should have what to deal with hazardous spills?
What do skin and eye exposures usually require if exposed to hazardous materials?
Every pharmacy should have a spill kit available. The spill kit is used to absorb chemicals. For large spills call the Poison Control Center for assistance with cleaning up the spill.
Skin exposures require the removal of clothing around the affected area and to wash the area with cool water for 15 minutes.
Eye exposures require flushing the eyes for 15 minutes. Contact lenses should be removed prior to the eye wash
What are the OSHA recommendations for reducing the risk of blood borne pathogen transmission?
Require bloodborne pathogen training for all at risk employees
Wear PPE whenever there is a reasonable risk of exposure
Wash hands prior to and after patient care, after removal of PPE and after contact with blood or potentially infectious material
Use safer or needleless devices to reduce needle stick injuries or sharp exposures
Avoid the splashing, spraying or spattering of body fluids
Use labeled biohazard containers or red bags labeled as “Infectious Waste” for the transfer or disposal of contaminated materials
Use approved disinfectants on contaminated items and equipment before reuse
Offer hepatitis B vaccinations to all employees that are at potential risk of exposure
Prohibit eating and drinking in work areas in which there is a risk of exposure
Ensure that a post exposure evaluation and a follow up plan are in place to address exposures
What steps should be followed after a bloodborne pathogen exposure?
1) Wash or irrigate the site of exposure
a. For needlestick injuries, wash the puncture site with soap and water.
b. For skin exposures, wash the exposure site with soap and water.
c. For exposure into the nose or mouth, flush the area with water.
d. For eye exposures, irrigate eyes with clean water, saline or sterile irrigation fluid. If the department has an eye wash station, this is best for eye irrigation.
2) Report exposure to the instructor, preceptor or supervisor.
3) Seek medical evaluation as soon as possible. Post exposure treatments help prevent infection.
DEA Form 222 is used for ….?
The Electronic Controlled Substance Ordering System (CSOS) is the electronic equivalent of….?
The CSOS allows the purchaser to purchase...?
used to order, sell or transfer Schedule I and II controlled substances
DEA Form 222
Sched I-II drugs. You can also use this system to order III-V but it is not required
Who can complete DEA Form 222? How can these forms be filled out?
Can C-II orders be partially filled by the supplier?
How long must a copy of DEA Form 222 be kept?
Only the registrant and those authorized by power of attorney on behalf of the registrant can complete this form. Forms must be written in indelible ink or typewritten (may be electronic) and no changes or erasures are permitted
Orders may be partially filled by the supplier, but the remaining quantity must be shipped within 60 days
ALL DEA Forms must be stored at the pharmacy for 2 years from the date of execution
What is DEA Form 224 for?
controlled substance registration for manufacturers, distributors, or dispensers that wish to handle controlled substances.
What is DEA Form 41 for?
controlled substance destruction/disposal confirmation form
What is DEA Form 106 for?
to report theft or loss of controlled substances
What are the 3 types of ways in which prescriptions can be filed?
1) 3 separate prescription files
Schedule 2 substances
Schedule 3-4 and five substances
All non-controlled drugs
2) 2 separate prescription files
Schedule 2 substances
All other drugs dispensed
If schedules III-V prescriptions are mixed with the non-controlled drugs, >/= 1 inch red letter C must be used in all scheduled prescriptions to identify them from the rest of the non-controlled prescriptions in the file
3) Electronic Prescription records
All records of prescriptions created, signed, transmitted, and received electronically must be retained for two years from the date of creation
Electronic records must be easily read
Records for controlled substances should be retrievable from all other records
Inventory and ordering (such as DEA form 222) must be kept separate from other records.
Can Prescription records be kept at a central location instead of at the pharmacy?
Can inventories and executed DEA forms be kept at a central location?
Yes, prescription records can be kept at a central location so long as the DEA is notified beforehand and the records remain retrievable within 2 days.
No, inventories and executed DEA forms must be kept at the pharmacy and CANNOT be sent to a central location
How long must records about controlled substances must be kept?
All records for any controlled substances must be kept on file for at least 2 years.
Can a Schedule III-V controlled medication prescription be accepted by facsimile transmission (Fax) ?
Yes
Can a Schedule II controlled medication prescription be accepted by facsimile transmission (Fax) ?
In General = NO, except
A patient is a resident of a long-term care facility
A patient is enrolled in Hospice
A narcotic pain therapy drug is prescribed for a terminally ill patient.
The medication is parenteral, IV, IM, SC or intraspinal infusion that is going to be compounded for direct administration to a patient
The prescriber, patient, or patient’s agent faxes a valid, signed prescription to the pharmacy in order to expedite pre-dispensing preparations.
Can Schedule III-V controlled medications be accepted via verbal order or voicemail?
Schedule III-V controlled medications CAN be accepted by verbal order or voicemail.
Can Schedule II controlled medications be accepted via verbal order or voicemail?
Verbal orders for Schedule II controlled substances are only permitted in emergency situations under the following conditions:
The drug is required for immediate administration
Any drug that is of a lesser schedule will not be an effective alternative.
The pharmacist must verify the authenticity of the prescriber issuing the verbal order.
The quantity prescribed in the verbal order should cover the emergency period Only.
The information given in the verbal order must be immediately reduced to writing by the pharmacist.
The hard copy prescription from the prescriber must be signed in indelible ink, state “Authorization for Emergency Dispensing”, and include the date of the verbal order.
An original hard copy prescription must be postmarked or delivered to the pharmacy within 7 days of the verbal order.
If the prescription is not received or postmarked within 7 days the pharmacy must notify the DEA.
Which Schedule of Drugs requires a continuous inventory (a record of the current inventory, drugs received, and drugs dispensed) ?
Is required for Schedule II medications
NOT required for Schedules III-V
What are the 3 main ways that controlled substances can be disposed?
1) They can be transferred or returned to a reverse distributor authorized to possess controlled substances. The reverse distributor will issue a DEA form 222 for the transfer/return schedule II drugs.
2) The controlled substances may be destroyed in the presence of an authorized member of the DEA drug control division or law enforcement. DEA form 41 detailing the controlled substances disposed of must be filled out and then sent to the DEA.
3) Hospitals or facilities that are licensed to administer medications may obtain a blanket authorization from the FDA to immediately destroy controlled substances on site in the presence of two authorized employees. A record of the destruction should be recorded using DEA form 41 and the form should be submitted to the DEA within 10 days of the destruction.
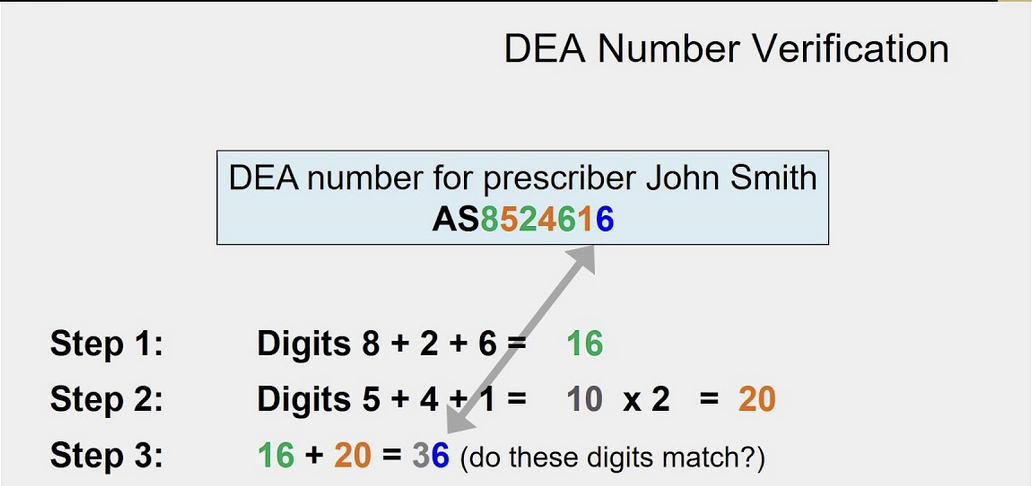
How do you Verify a DEA Number?
add the 1st, 3rd, and 5th digits
add the 2nd, 4th, and 6th digits. Double it
add the two totals together. the second digit is the check digit
1st letter represents the type of practice or institution
2nd letter represents the first letter of the practitioner's last name
What do certain Letters mean for DEA Numbers?
A, B,F- physicians, dentists, etc
G- Department of Defense contractors
M- Mid-level practitioners (NPs, PAs, etc.)
P- Manufacturers, distributors
R- Manufacturers, distributors, researchers
X- Prescribers authorized to prescribe under a narcotic treatment program
How old does a patient have to be to buy OTC pseudoephedrine?
18yrs or older
How much pseudoephedrine can someone buy?
Daily limit = 3.6g
30-day limit = 9g
If mail order/online Pharm = 7.5g
What must the pharmacy do when selling pseudoephedrine?
The pharmacy must scan the purchaser's government-issued photo ID, report the purchase to New York's tracking system.
The electronic record of the sale must have photo ID number, date and time of transaction, product name, quantity sold, purchaser’s name, address, DOB and purchaser’s signature
What must the purchaser do when buying pseudoephedrine?
The purchaser must sign that they understand the purchase limits and penalties for improper purchase.
How long must records of the sale of pseudoephedrine be kept?
2 years
What is a Behind-the Counter (BTC) medication?
BTC medications are OTC medications that must be kept behind the pharmacy counter or in a locked cabinet because of their potential for abuse. The purchaser must be at least 18years old and show photo-ID prior to purchasing.
What is a REMS Program?
A Risk Evaluation and Mitigation Strategy (REMS)- is a drug safety program required by the FDA for certain medications that pose serious safety concerns
REMS programs are designed to ensure safe and proper use of a medication
Which medications require a REMS?
Accutane (isotretinoin)
Clozaril (clozapine)
Pomalyst (pomalidomide)
Revlimid (lenalidomide)
Thalomid (thalidomide)
What is a The Drug Supply Chain Security Act (DSCSA) ?
was enacted in 2013 as an effort to reduce the prevalence of counterfeit drugs.
This act established a national tracing system for medications called a drug pedigree.
Tracking begins at the manufacturing facility where the drug is produced.
What information does a drug pedigree contain?
The types of tracking information contained in the drug pedigree include the name, strength and dosage of the product; NDC number; container size; number of containers; product lot numbers; transaction date; shipment date; name and address of the seller and purchaser and transaction history.
How long are Pharmacies required to drug pedigree info?
Pharmacies are required to obtain drug pedigree info and maintain drug pedigrees for 6 years.
List the details of Accutane (isotretinoin)’s REMS program
Isotretinoin’s REMS program is called iPledge
Prescriptions for isotretinoin may be dispensed within 7 days of a negative pregnancy test.
A maximum of 30days supply can be dispensed at a time.
Patients who register with iPledge program must:
Receive medication counseling from a pharmacist and sign a consent form
Monthly pregnancy tests
Use 2 methods of contraception throughout treatment and for 30 days after treatment
What is the difference between a Drug and a Device?
Drug- any compound recognized in the official United States pharmacopeia (USP), National Formulary (NF) or The Homeopathic Pharmacopeia of the United States.
Device- an instrument, apparatus, implement, machine, implant, individual reagent or other component, part or accessory that is recognized in the official National Formulary or USP.
What is a Recall?
A recall is a voluntary removal or correction of a product that is on the market because it is adulterated, misbranded, or in violation of the FDA approval.
A recall can be initiated by the manufacturer or the FDA.
A Class I recall is defined as…?
a situation in which the FDA deems there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.
In the case of a Class I recall, the pharmacy MUST...?
make a reasonable attempt to notify all patients that have been prescribed and who are currently taking such recalled drug.
The contact attempt can be done by phone or by mail within 3 days of the pharmacy being notified by the FDA, a manufacturer, a wholesaler or by other notice of such recall.
A Class II recall is defined as…?
Drugs or devices that could cause temporary reversible effects or have a small chance of causing serious adverse effects if administered
All affected drug stocks at the pharmacy must be isolated, but a notice to patients is not usually required.
A Class III recall is defined as…?
Drugs or devices that are unlikely to cause any adverse health effects if administered. Usually results from a packaging or labeling violation.
What does the Omnibus Budget Reconciliation Act of 1990 (OBRA 90) require pharmacists to do?
Under OBRA-90 a pharmacist must conduct a review of drug therapy prior to dispensing each prescription.
OBRA-90 also requires pharmacists to offer each Medicaid patient counseling on their prescriptions.
What is HIPAA?
How are providers able to obtain PHI?
· HIPAA is a US federal law enacted in 1996 that sets national standards for protecting sensitive patient health information. Patient health information must be stored securely and disposed of properly.
· All health care providers are assigned a unique 10-digit national provider identifier (NPI) number that allows protected health information to be transferred more securely
When is patient authorization required for PHI release?
Patient authorization is required to disclose information for marketing and other disclosures not required for treatment.
When it comes to PHI, patients have the right to…?
To obtain a copy of their PHI.
Patients themselves must sign and date an authorization form, which is good up to a year, and the pharmacy must comply within 30 days.
To be notified of an information breach
Pharmacies must notify patients within 60 days of an information breach
To know about privacy practices in the pharmacy
A notice of privacy practices must be posted in the pharmacy and must be available to patients. This notice must include how the pharmacy intends to use, disclose and protect the patients’ health information as well as the patients right and information on how to file a complaint
When can PHI can be disclosed without patient authorization?
for refill reminders, disease management programs, communications to the patient, general health promotion, court orders or subpoenas or for serious health threats (i.e. child abuse).
Who can obtain a patient’s medical records?
Another family member or relative is NOT authorized to pick up a patient’s medical records unless they are authorized by power of attorney to do so.
If a child is younger than 18 years of age, their parent or legal guardian may obtain a copy of their PHI.
What is a Patient Package Insert (PPI) vs a Consumer Medication Information (CMI)?
Patient Package Insert (PPI)- is a detailed document included with a prescription drug that contains technical information intended primarily for healthcare professionals, including potential side effects, dosage instructions, and contraindications
Consumer Medication Information (CMI)- a document written in plain language for patients, providing basic information about a medication, typically developed by an organization outside the drug company; CMIs are not FDA approved
A CMI must be provided with…?
A PPI must be provided with… ?
The FDA mandates that useful patient information be provided to patients with each new prescription
The FDA requires patient package inserts (PPIs) to be provided at the pharmacy for only two drug categories: estrogens and oral contraceptives.
What are MedGuides and for whom are they intended?
written patient information mandated by the Federal Drug Administration (FDA) for select high-risk drugs; also known as a patient medication guide.
When performing a transfer, the TRANSFERRING pharmacist must record....
1) Name, Address, and Telephone # of the receiving pharmacy
2) Name of the pharmacist receiving
3) Name of the pharmacist transferring
4) Day of transfer
When performing a transfer, the RECEIVER pharmacist must record....
1) Refill transfer on the face of the hard copy of Rx
2) Date of the original prescription
3) Most recent filling of prescription
4) Name and address of the transferring Pharmacy
5) Original prescription number
6) Name of pharmacist transferring
7) Name of pharmacist receiving