3.Chemical Reactions
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Reactants
Substances present at the start of a chemical reaction that participate in the reaction.
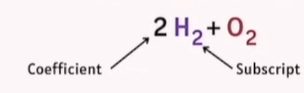
Coefficient
The number placed before a molecule that indicates how many molecules are involved (e.g., 2H₂ means two molecules of hydrogen).
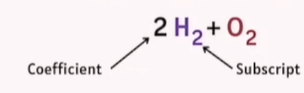
Subscript
The small number written after an element that shows how many atoms of that element are in the molecule (e.g., H₂ means two atoms of hydrogen).
Irreversible Reaction
A chemical reaction that cannot easily go back to the original substances.
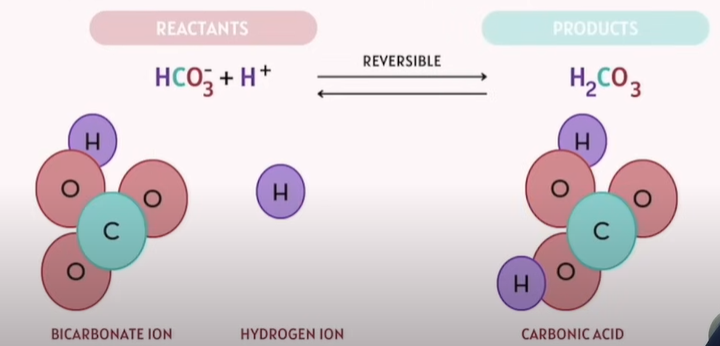
How a reversible reaction looks like?
Note: The arrows in the middle pointed both ways.
If we add more reactants, it will push the reactants towards the product side and vice versa.
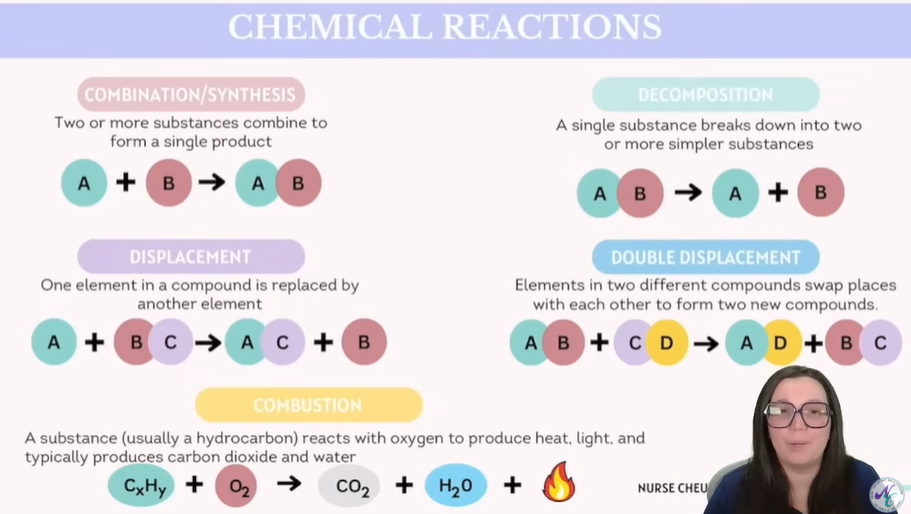
5 Types of Chemical Reactions - Overview
Reaction Type | Description | General Formula | Example (not in image) |
|---|---|---|---|
Combination / Synthesis | Two or more substances combine to form one product. | A + B → AB | 2Na + Cl₂ → 2NaCl |
Decomposition | A single compound breaks down into two or more simpler substances. | AB → A + B | 2H₂O → 2H₂ + O₂ |
Single Displacement | One element in a compound is replaced by another element. | A + BC → AC + B | Zn + HCl → ZnCl₂ + H₂ |
Double Displacement | Elements in two different compounds swap places with each other to form two new compounds. Note: The inner elements combine and the outer elements combin to form new compounds. | AB + CD → AD + CB | AgNO₃ + NaCl → AgCl + NaNO₃ |
Combustion: Hallmark sign of combustion: The second reactant is oxygen, and the products are CO2 and H20 | A substance (usually a hydrocarbon)reacts with oxygen to produce CO₂, H₂O, and energy (heat/light). | CₓHᵧ + O₂ → CO₂ + H₂O + energy | CH₄ + 2O₂ → CO₂ + 2H₂O + 🔥 |
5 Types of Chemical Reactions - Quiz
Flashcard Question (Front) | Flashcard Answer (Back) |
|---|---|
What happens in a combination/synthesis reaction? | Two or more substances combine to form a single product (A + B → AB). |
What happens in a decomposition reaction? | A compound breaks into simpler substances (AB → A + B). |
What happens in a single displacement reaction? | One element replaces another in a compound (A + BC → AC + B). |
What happens in a double displacement reaction? | Two compounds exchange elements (AB + CD → AD + CB). |
What is a combustion reaction? | A hydrocarbon reacts with O₂ to produce CO₂, H₂O, and heat/light. |
What are the products of a combustion reaction? | Carbon dioxide (CO₂), water (H₂O), and heat or light energy. |
In which reaction does energy in the form of heat and light appear? | Combustion. |
Give an example of a synthesis reaction. | 2Na + Cl₂ → 2NaCl. |
Give an example of a double displacement reaction. | AgNO₃ + NaCl → AgCl + NaNO₃. |
📝 Additional TEAS Exam Notes (Not in Images)
Concept | Explanation |
|---|---|
Recognizing Reaction Types by Formula | On the TEAS, you may be asked to identify reaction types just from the formula format (e.g., A + B → AB = Synthesis). |
Balancing Reaction Equations | TEAS will test your ability to balance chemical equations so that atoms on both sides are equal. |
Physical vs Chemical Changes | Chemical changes involve new substances forming. Reaction types like decomposition, combustion, and synthesis are all chemical changes. |
Energy Transfer in Reactions | Endothermic (absorbs energy) vs Exothermic (releases energy—like combustion). TEAS may ask which reaction types release or absorb heat. |
State Symbols | Know these: (s) = solid, (l) = liquid, (g) = gas, (aq) = aqueous (dissolved in water). E.g., NaCl(aq) + AgNO₃(aq) → AgCl(s) + NaNO₃(aq). |
What is Diatomic Molecules?
🔹 Most commonly, on the TEAS, this refers to diatomic elements:
Elements that naturally exist as pairs of the same atom when in their pure form.
🧪 The 7 Diatomic Elements (memorize for TEAS):
Element | Formula | Mnemonic |
|---|---|---|
Hydrogen | H₂ | |
Nitrogen | N₂ | |
Oxygen | O₂ | |
Fluorine | F₂ | |
Chlorine | Cl₂ | |
Bromine | Br₂ | |
Iodine | I₂ |
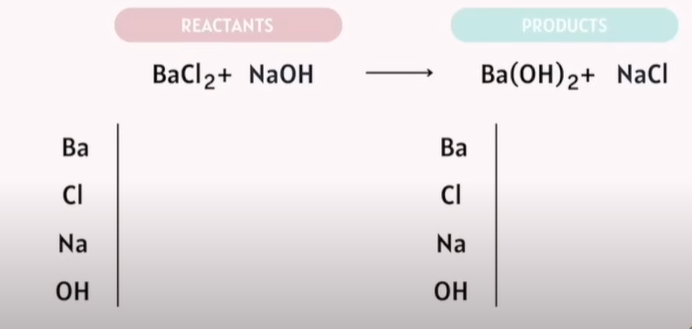
Balancing Chemical Reactions
Do practice!!
The goal: Reactants are the same exact number as your products.
Tips:
Save Oxygen and Hydrogen balancing for last.
-Save Oxygen to the last last!!!!!
If Polyatomic atoms are on the same side. Do not separate them.
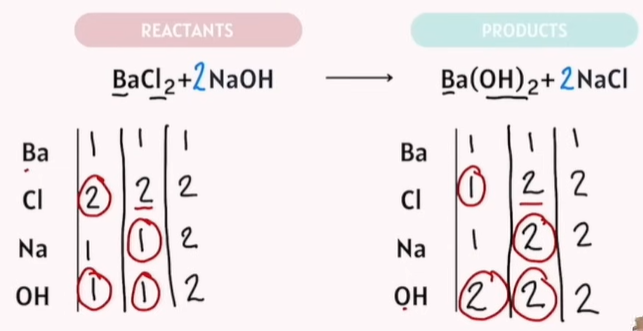
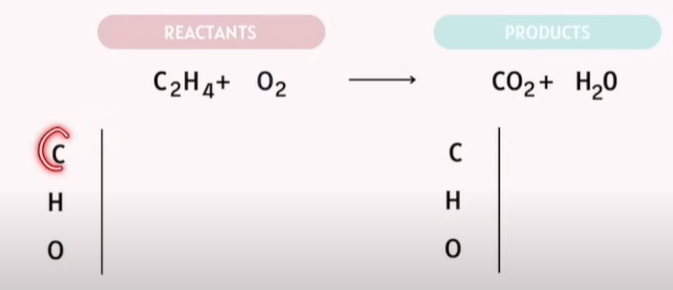
Balancing Chemical Reactions Practice
The goal: Reactants are the same exact number as your products.
Tips:
Save Oxygen and Hydrogen balancing for last.
-Save Oxygen to the last last!!!!!
If Polyatomic atoms are on the same side. Do not separate them.
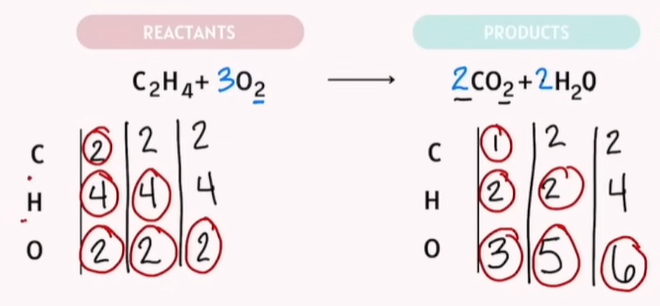
What is Polyatomic atoms?
🧪 What Are Polyatomic Ions?
Definition:
A polyatomic ion is a charged group of two or more atoms that are covalently bonded and act as a single ion in chemical reactions.
"Polyatomic" = many atoms
"Ion" = has a charge (positive or negative)
🔋 Charge:
The group has an overall charge even though the atoms are bonded together.
Example: NO₃⁻ (nitrate) is made of nitrogen and oxygen but behaves like a single unit with a -1 charge.
🧠 Common Polyatomic Ions to Know for TEAS
Name | Formula | Charge |
|---|---|---|
Ammonium | NH₄⁺ | +1 |
Hydroxide | OH⁻ | -1 |
Nitrate | NO₃⁻ | -1 |
Sulfate | SO₄²⁻ | -2 |
Carbonate | CO₃²⁻ | -2 |
Phosphate | PO₄³⁻ | -3 |
Bicarbonate | HCO₃⁻ | -1 |
Acetate | C₂H₃O₂⁻ or CH₃COO⁻ | -1 |
🔍 Key TEAS Points
They appear in ionic compounds (e.g., NaNO₃ is made of Na⁺ and NO₃⁻).
You must keep them together as a unit when writing formulas or balancing equations.
Use parentheses when you need more than one in a compound (e.g., Ca(NO₃)₂).
Moles
Unit of measurement that is the amount of a pure substance containing the same number of chemical units.
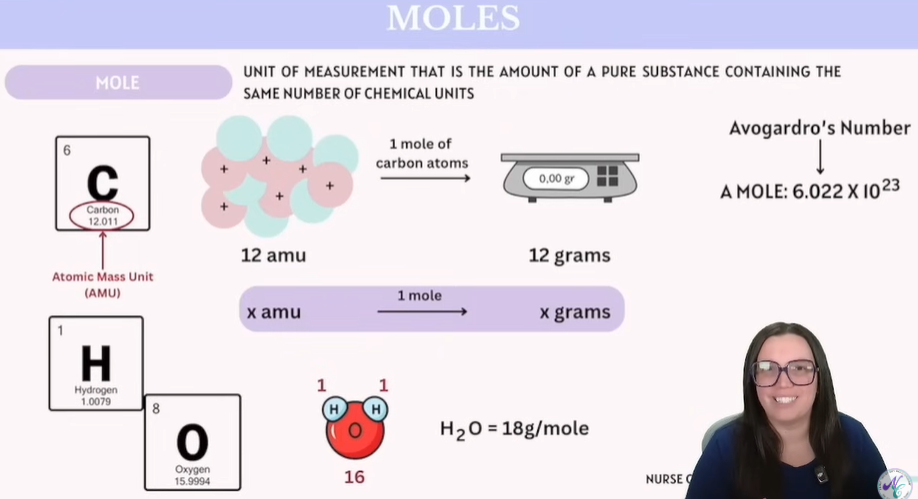
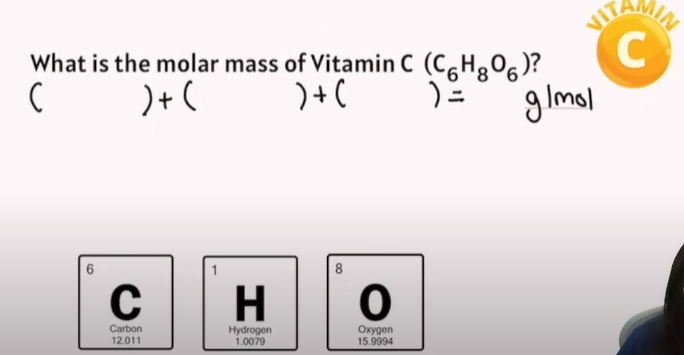
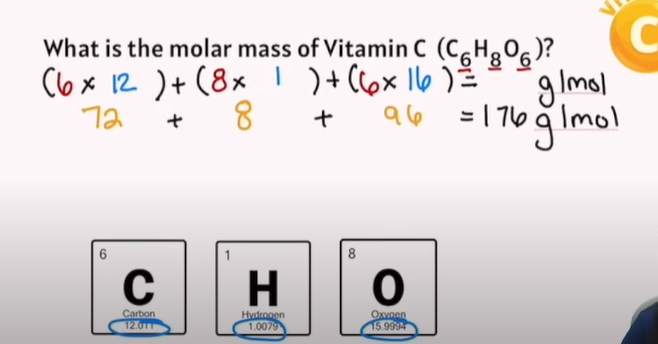
Practice Example of Moles calculations:
Collision Theory Table – Factors That Affect Chemical
Factor | Description | TEAS Key Term |
|---|---|---|
Collision Theory | Particles must collide with sufficient energy to cause a chemical reaction. | Collision Theory |
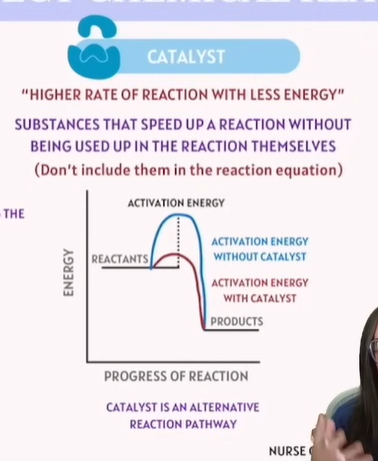
Activation Energy | Minimum amount of energy required for a reaction to occur. | Activation Energy |
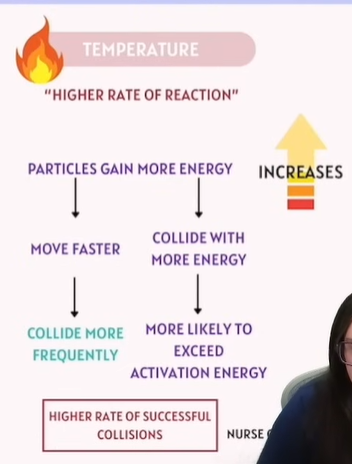
1. Energy of Particles | The more kinetic energy particles have, the more likely they will overcome activation energy. | Temperature |
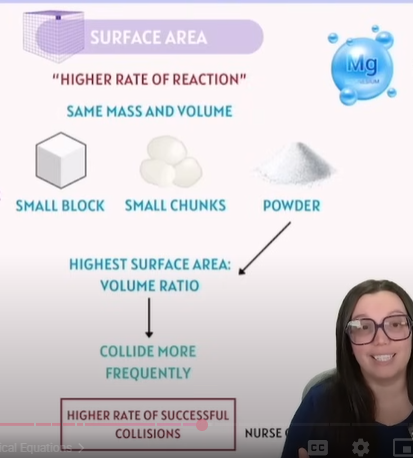
2. Frequency of Collisions | The more often particles collide, the higher the chance for successful collisions (reactions). | Concentration / Surface Area |
Factors that affect Chemical Reactions - Temperature
Factors that affect Chemical Reactions - Concentration and Pressure

Factors that affect Chemical Reactions - Surface Area
Factors that affect Chemical Reactions - Catalyst
Higher rate of reaction with less energy!
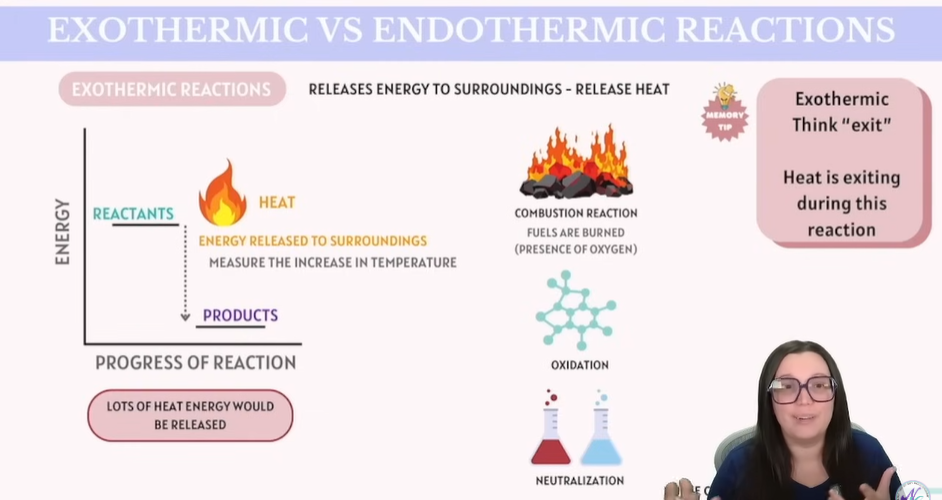
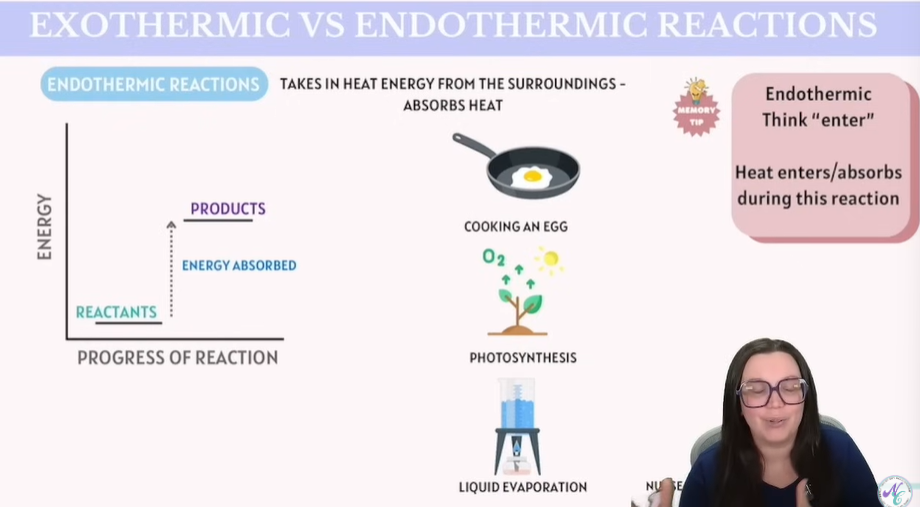
Exothermic vs Endothermic Reactions
Exothermic Reactions - Release energy to surroundings - Release HEAT !!
Tip: Exothermic = Think Exit
Heat is exiting during this reaction.
Examples:
Combustion Reaction
Oxidation
Nutralizing
Exothermic vs Endothermic Reactions
Endothermic Reactions - Absorb energy from surroundings - Absorb HEAT !!
Tip: Endothermic = Think Enter
Heat is absorbed during this reaction.
Examples:
Cooking an egg
Photosynthesis
Liquid Evaporation
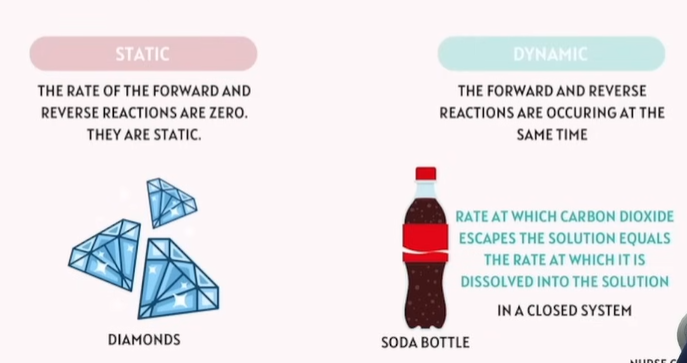
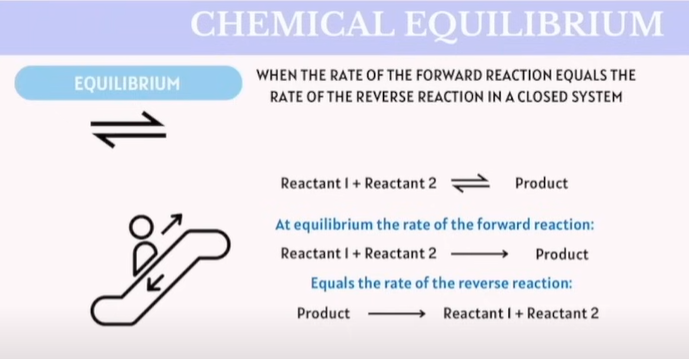
Chemical Equilibrium
When the rate of the forward reaction reaction equals the rate of the reverse reaction in a close system.
Two Type of Chemical Equilibrium
Static
Dynamic