3.2..2 HYDROCARBONS (ALKANES)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is hydrogenation, and how does it produce alkanes?
Definition: Addition of hydrogen to unsaturated alkenes with C=C bonds to form saturated alkanes.
Mechanism:
Reactants: Alkene + H₂ gas.
Conditions: Heat and a finely divided Pt/Ni catalyst (high surface area increases reaction rate).
Example:
Reaction: Butene + H₂ → Butane (with Pt/Ni catalyst).
Applications:
Used in the food industry to partially hydrogenate vegetable oils to make margarine (raises melting point).
Cracking of Hydrocarbons
Definition: Breaking large hydrocarbons into smaller alkanes and alkenes.
Mechanism:
Reactants: Large hydrocarbon chains (e.g., decane).
Conditions: Heat in steel chambers with Al₂O₃ catalyst (oxygen-free to prevent combustion).
Example:
Reaction: Decane → Octane + Ethene.
Products:
Smaller alkanes: Fuel-grade hydrocarbons.
Alkenes: Used in polymer production.
Comparison of Hydrogenation and Cracking
Hydrogenation:
Type: Addition reaction.
Energy: Exothermic.
Uses: Food industry, fuel refinement.
Cracking:
Type: Thermal decomposition.
Energy: Endothermic.
Uses: Fuel production, chemical synthesis.
What is complete combustion, and what are its products?
Complete combustion occurs when alkanes are burnt in an excess of oxygen. During this reaction, all carbon and hydrogen are fully oxidized, producing:
Carbon dioxide (CO₂)
Water (H₂O)
Example: Complete combustion of octane: C₈H₁₈ + 12.5O₂ → 8CO₂ + 9H₂O
Key Features:
Requires plenty of oxygen.
Releases a significant amount of energy, making alkanes useful as fuels.
What is incomplete combustion, and what are its risks?
Incomplete combustion occurs when alkanes are burnt in a limited oxygen supply. This reaction partially oxidizes carbon, producing:
Carbon monoxide (CO): A toxic gas.
Water (H₂O) Example: Incomplete combustion of octane: C₈H₁₈ + 8.5O₂ → 8CO + 9H₂O
Key Risks of Carbon Monoxide:
Binds to haemoglobin, preventing oxygen transport in the blood.
Causes symptoms like dizziness and loss of consciousness; prolonged exposure can be fatal.
Odourless and hard to detect.
Occurrence: Common in car engines due to limited oxygen.
What is free-radical substitution, and what is needed for the reaction to occur?
Free-radical substitution is a reaction in which a hydrogen atom in an alkane is substituted by a halogen (chlorine/bromine).
Requirement: Ultraviolet light (UV light) is essential for initiating the reaction, as alkanes are generally unreactive.
Steps:
Initiation: Formation of halogen radicals.
Propagation: Chain reaction producing products and regenerating radicals.
Termination: Radicals combine to end the reaction.
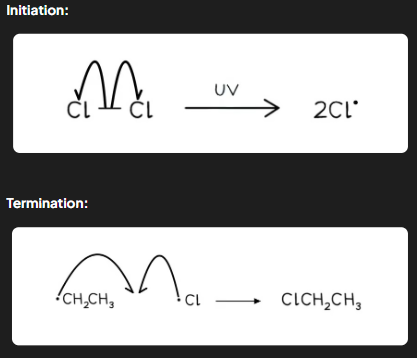
What happens during the initiation step of free-radical substitution?
The covalent bond in a halogen molecule (Cl-Cl or Br-Br) is broken by UV light energy, causing homolytic fission.
Reaction: Cl₂ → 2Cl•
Homolytic Fission: Each halogen atom takes one electron from the bond, forming two highly reactive radicals.
Key Feature: The radicals formed will initiate further reactions by attacking alkanes.
Describe the two main reactions in the propagation step of free-radical substitution.
During propagation, radicals react with alkanes to create a chain reaction:
Step 1: A halogen radical (e.g., Cl•) reacts with the alkane, breaking a C-H bond and producing an alkyl radical. Example: CH₄ + Cl• → •CH₃ + HCl
Step 2: The alkyl radical reacts with a halogen molecule, forming a halogenoalkane and regenerating the halogen radical. Example: •CH₃ + Cl₂ → CH₃Cl + Cl•
Key Feature: The regenerated halogen radical continues the chain reaction, repeating the cycle.
What occurs during the termination step of free-radical substitution?
In termination, two radicals combine to form a stable product, ending the chain reaction. Examples of Termination Reactions:
Methyl radical + Chlorine radical: •CH₃ + Cl• → CH₃Cl
Methyl radical + Methyl radical: •CH₃ + •CH₃ → CH₃CH₃
Chlorine radical + Chlorine radical: Cl• + Cl• → Cl₂
Key Feature: The removal of radicals stops the reaction.
What happens if there is excess halogen present during free-radical substitution?
Excess halogen leads to multiple substitutions, replacing all hydrogens in the alkane with halogens. Example of Multiple Substitutions with Methane:
First Substitution: CH₄ → CH₃Cl CH₃Cl + Cl• → •CH₂Cl + HCl •CH₂Cl + Cl₂ → CH₂Cl₂ + Cl•
Second Substitution: CH₂Cl₂ → CHCl₃ CHCl₃ + Cl• → •CCl₃ + HCl •CCl₃ + Cl₂ → CCl₄ + Cl•
Key Note: Multiple substitution makes free-radical substitution unsuitable for preparing specific halogenoalkanes, as mixtures of products are formed.
How are arrows used to depict the free-radical substitution mechanism?
Initiation: Uses half-headed (fish-hook) arrows to show the movement of a single electron.
Propagation: Continues with fish-hook arrows showing radical generation and product formation.
Termination: Fish-hook arrows depict radicals combining to form stable molecules.
Exam Tip: Avoid using equations that reform the original halogen (e.g., Cl₂ → 2Cl• → Cl₂) when asked about the termination step, as these may be ignored in mark schemes.
What is crude oil, and how is it processed to obtain useful fractions?
Crude oil is a mixture of hydrocarbons, including alkanes, cycloalkanes, and arenes (compounds with a benzene ring).
Extraction: Obtained from the earth through drilling.
Transportation: Taken to oil refineries.
Fractional Distillation: A process in which hydrocarbons are separated based on boiling points, producing fractions with similar boiling points.
High Demand Fractions: Smaller hydrocarbon fractions (e.g., gasoline) are in high demand compared to heavier fractions.
What is cracking, and why is it performed on heavier crude oil fractions?
Cracking is the process of breaking larger, heavier hydrocarbons into smaller, more useful alkanes and alkenes of lower relative formula mass (Mr). Purpose:
Converts excess heavy crude oil fractions into smaller hydrocarbons in high demand. Process:
Large hydrocarbon molecules are fed into a steel chamber.
Heated to high temperatures.
Passed over an aluminium oxide (Al₂O₃) catalyst.
Oxygen is excluded to prevent combustion into CO₂ and H₂O.
What are the products of cracking, and what are their uses?
Cracking produces:
Alkanes: Low-molecular mass hydrocarbons, highly useful as fuels due to their energy release during combustion.
Example: Octane, used in gasoline.
Alkenes: Reactive hydrocarbons with double bonds, used as feedstock in chemical industries.
Example: Ethene, used as a monomer in polymerisation reactions to create plastics.
What are the broader environmental impacts of pollutants from car engines?
Carbon Monoxide: Toxic to humans, interferes with oxygen transport.
Oxides of Nitrogen:
Cause acid rain, which corrodes buildings and harms aquatic and plant life.
Contribute to smog, reducing air quality.
VOCs and PAN:
Smog formation harms respiratory health and reduces visibility.
Damage to ecosystems and plant life.