unit 3: thermal physics
1/34
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
internal energy
contained within a system or body (does NOT include environment, speed etc.), the sum of the random distribution of kinetic and potential energies of the objects atoms and molecules
heat
the transfer of thermal energy from one body to another as a result of a difference in temperature, changes the internal energy of a system, symbol for heat is Q, unit is Joules
Kelvin (K)
SI unit for temperature, ºC+273, objects cannot be at 0K because it means their particles are not moving
specific heat capacity (c)
energy required to raise the temperature of a unit mass by one K/ºC without a change of state, measured in Jkg^-1K^-1
specific heat equation: Q=mcΔT
Q=heat energy required in J
m=mass in kg
c=specific heat capacity in J Kg^-1K^-1
ΔT=change in temperature in K
thermal energy
the total random kinetic energy of the particles of an object
thermal equilibrium
no transfer of thermal energy occurs thus objects are at the same temperature
what allows for a mass to be heated without a change of state?
the specific heat equation
how do changes of state affect heat transfer and temperature?
during changes of state heat is transferred but there is no change in temperature so Q=mcΔT doesn’t work
latent heat
the heat transferred when there is a change of state
how is heat affected when a change of state from solid to liquid to gas occurs?
heat is taken from the environment
how is heat affected when a change of state from gas to liquid to solid occurs?
heat is released to the environment
what is the latent heat of fusion (Lf) for?
melting and freezing
what is the latent heat of vaporisation (Lv) for?
boiling and condensation
how are changes of state shown on a graph?
a phase change is a flat line on the graph of time against temperature
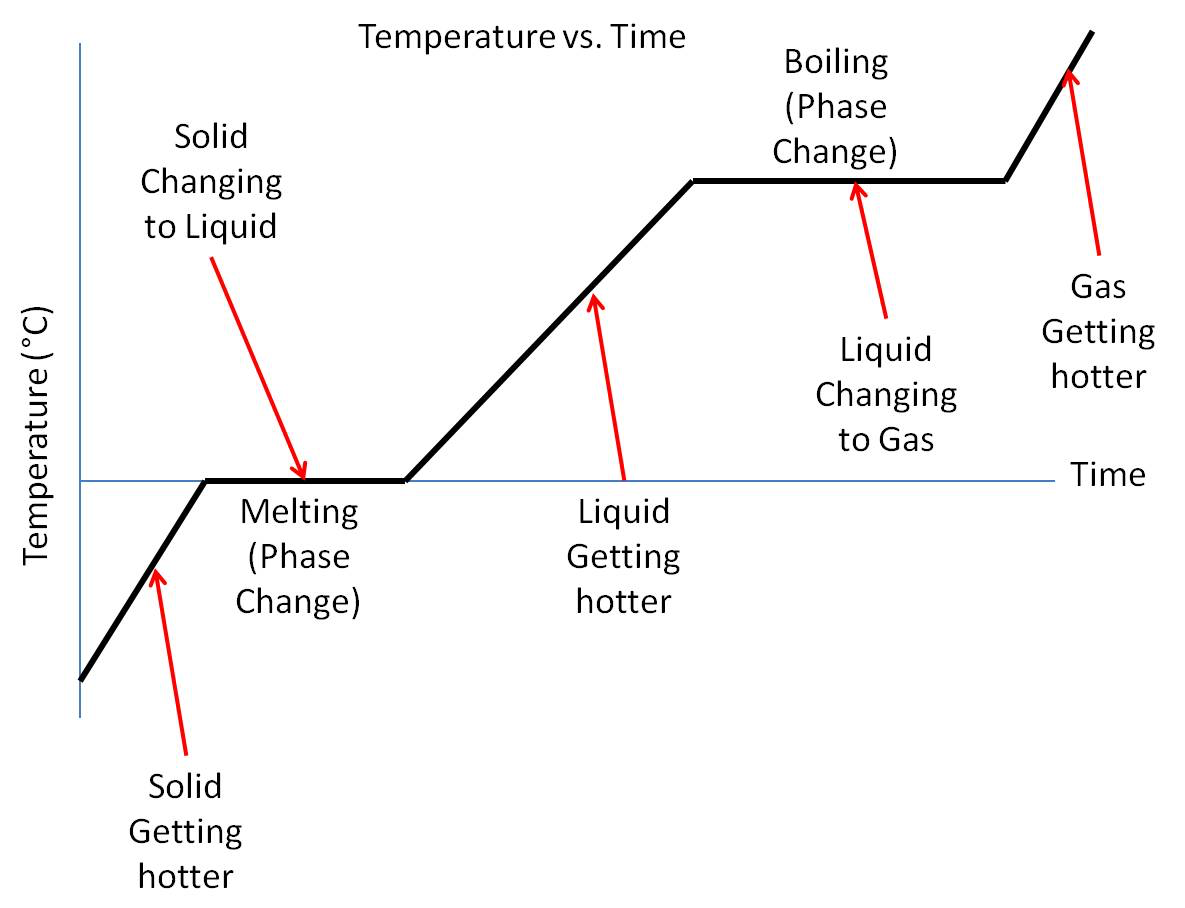
for water, does boiling or melting require more energy? what does this mean?
more energy is required to boil water than melt ice, this is shown as the melting line is shorter than the boiling line on the graph
therefore Lv > Lf for water
how can the total latent heat needed for a change of state be calculated?
L is measured in Jkg-1 so must be multiplied by the amount of kilos for the total latent heat needed
during a heat transfer when no heat is lost to the environment, what do we know about the heat energy required of the two objects?
when no heat is lost to the environment what is lost from one object is taken by the other object so |Q| will be the same but with opposite signs, what differs is the mass since Q is per kilogram
Boyle’s law
P1V1=P2V2
for gas at a constant temperature, pressure is inversely proportional to volume
the product of pressure and volume is a constant so if one changes the other one must too (unless the temperature changes)
if pressure is doubled volume halves, what you multiply one by you must divide the other one by
what is the SI unit for volume?
m^3, 1 litre = a cube with each side measuring 10cm =10^-3 m^3 so 1000 litres = 1m^3
what is the unit atm?
atm = atmospheres, 1atm =10^5 Pa, Pa=Nm^-2
Charles’ law
V1/T1=V2/T2
temperature must be Kelvins for this!
volume and temperature are proportional — if one goes up so does the other
what one is multiplied by the other must also be multiplied by
Gay-Lussac’s law
Gay-Lussac’s law: P1/T1=P2/T2
temperature must be Kelvins for this!
pressure and temperature are proportional — if one goes up so does the other
what one is multiplied by the other must also be multiplied by
combined gas law
(PV)(V/T)(P/T)= a constant
by simplifying and taking the square root: PV/T = a constant (for a constant mass)
Avogadro’s constant
V1/n1=V2/n2 (n = number of moles)
for two separate gases at the same temperature and pressure, equal volumes of gases contain the same number of moles, thus volume is independent of the identity of the gas
ratio of the volume to number of moles of two gases at the same temperature is always Avogadro’s constant
constant is 6.02x10^23, called N_a
one mole
a quantity of a substance that contains a number of particles equal to the Avogadro constant and whose mass in grams is equal to the molar mass of the substance
what is the amount of substance? how is it found?
it is the quantity of a substance measured in moles
number of molecules = number of moles x Avogadro’s constant, N=n x N_a
what is molar mass? how is it found?
the molar mass of the substance = mass of one mole of the substance
substance of a mass = M_s/M (substance mass/molar mass)
moles are ratios not physical units
what is an ideal gas?
a gas which obeys the ideal gas law for all values P, V and T
Boyle’s law: P1V1=P2V2
Charles’ law: V1/T1=V2/T2
Gay-Lussac’s law: P1/T1=P2/T2
what does the ideal gas law assume?
that only momentum changes when particles collide (hence ideal), all energy is conserved in the collision
what is the ideal gas equation? how can it be written?
PV/T = constant which is nR — the number of moles multiplied by the molar gas constant which is 8.31 J K^-1 mol^-1
therefore the ideal gas equation is PV=nRT with the temperature always in Kelvins
or written as PV=NkT (not in data booklet) which works as k=R/Na and k is Boltzmann’s constant (1.38x10^-23 J K^-1)
how are kinetic energy and temperature related?
kinetic energy is proportional to temperature
temperature is the average motion of things so doesn’t make sense for small numbers of particles but does for large numbers
this is a macroscopic result, only valid for a lot of particles
how is thermal energy found?
thermal energy = average random kinetic energies of the particles in a substance = 3/2 N k_B T
what is the total energy of ideal gases?
ideal gases have no potential energy whereas gases in reality have complex potential energy
for ideal gases total energy = internal energy (U)
how is the total energy of an ideal gas found?
U = 3/2 PV
U = total internal energy, as for ideal gases total = internal
Ek equation multiplied by N to make it total not average