General Chemistry I
1/42
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Nano (n)
10-9
Micro (µ)
10-6
Milli (m)
10-3
Centi (c)
10-2
Deci (d)
10-1
Deka (dk)
101
Hecto (h)
102
Kilo (k)
103
Mega (M)
106
Giga (G)
109
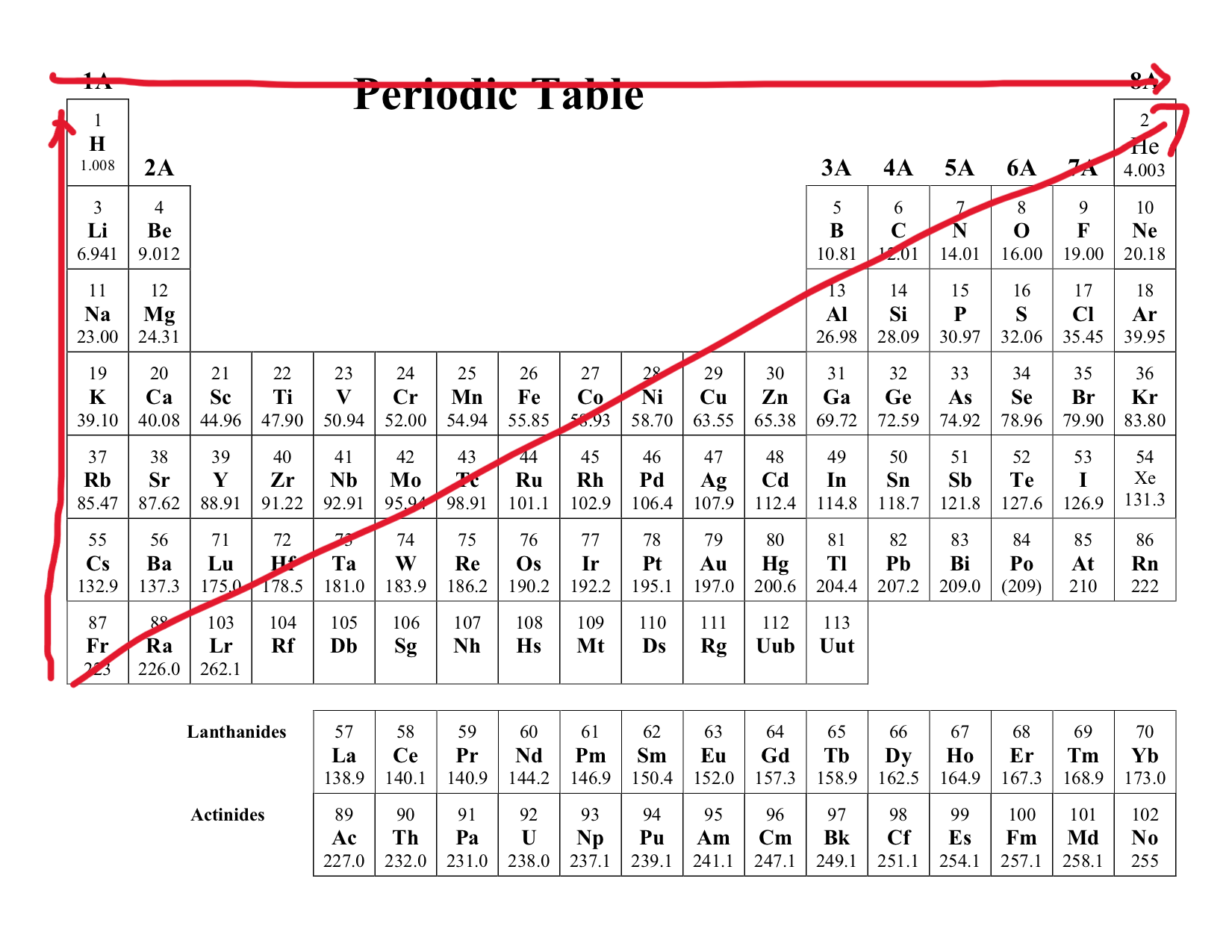
Period and Groups
This is the horizontal aspect of the periodic table. Ranging from left to right.
This is the vertical aspect of the period table. Top to bottom.
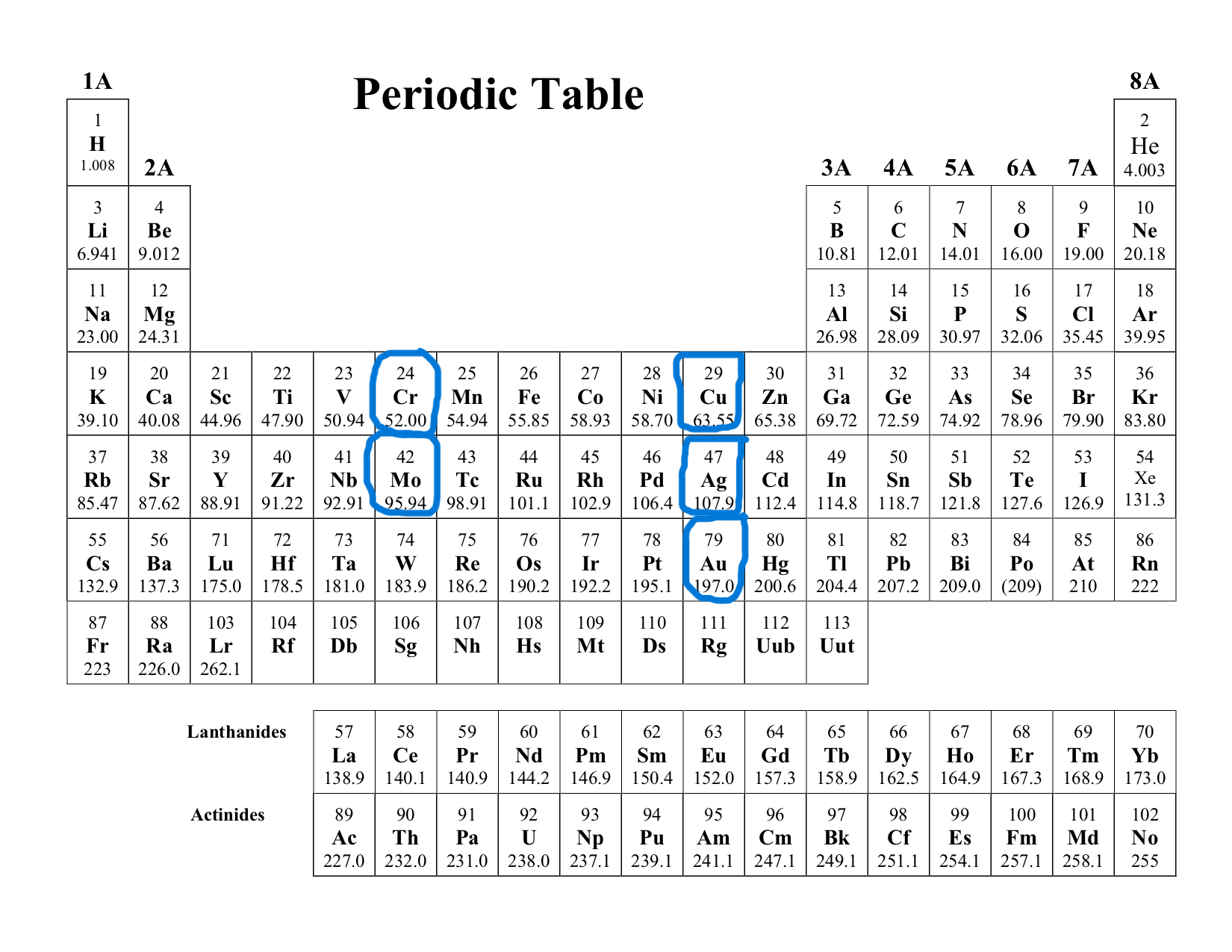
What are the exceptions to the electron configuration model?
Chromium (Cr)
Molibdenum (Mo)
Copper (Cu)
Silver (Ag)
Gold (Au)
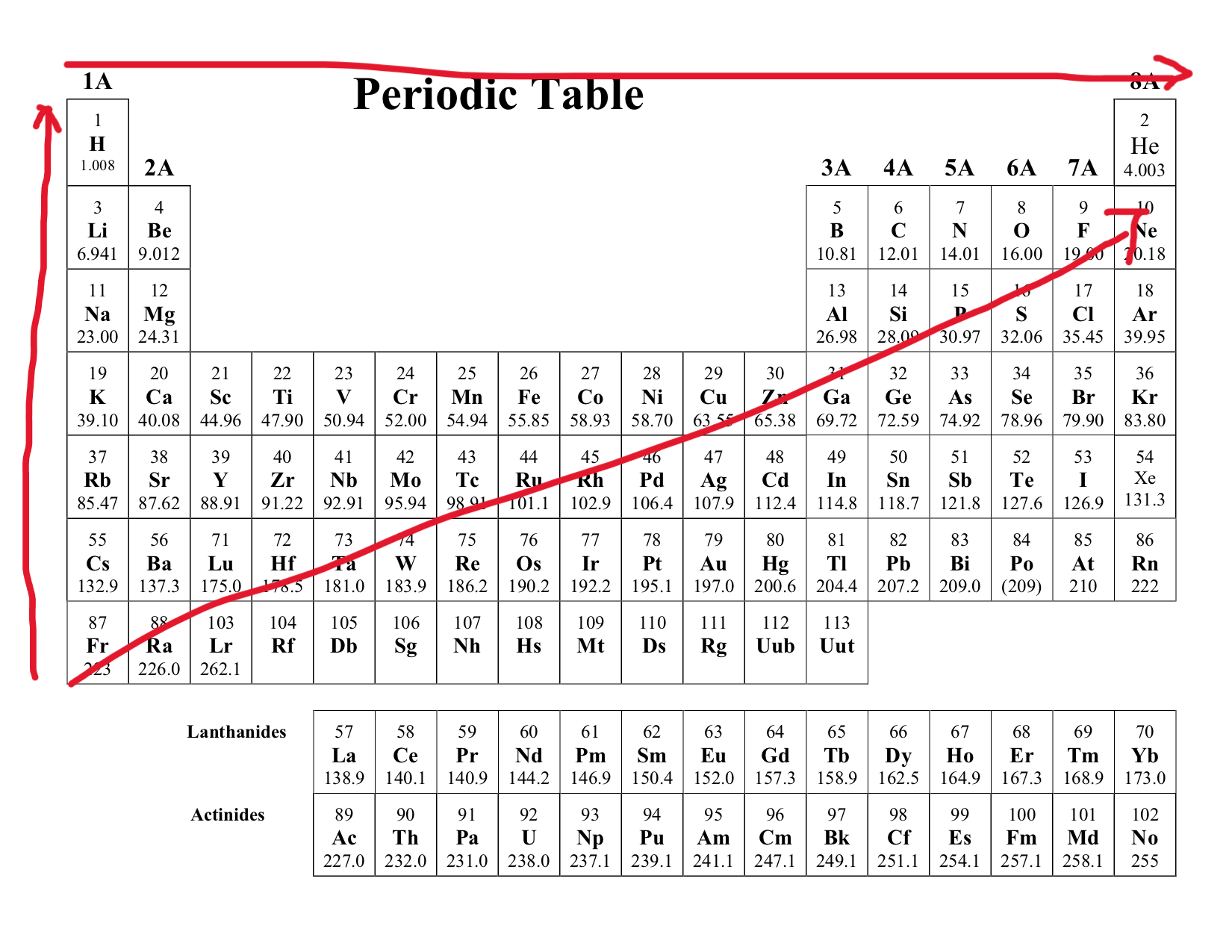
Electronegativity
Definition: This describes an atoms pull on another shared atom. The greater this is the stronger the pull.
Trend:
Increase as you move across a period (left → right)
Decrease as you move across a group (top → bottom)
As you move right of the group, this increases because the number of proton increases allowing more effective electron pull. But as you move down the period, this decreases because the protons get shielded by outer shell electron, making the electron pull less effective.
Exception: Noble Gases
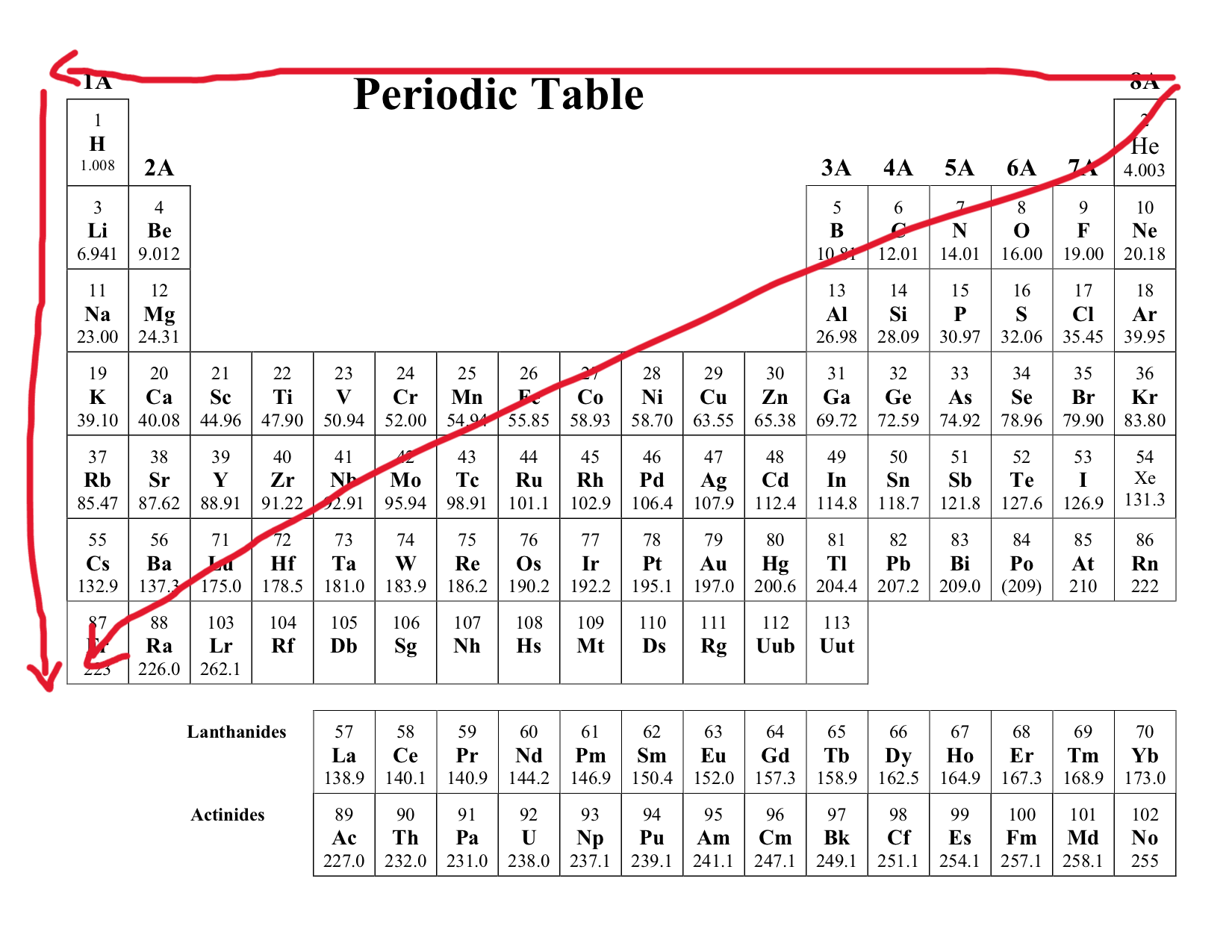
Atomic Size
Decrease as you move across a period (left → right)
Increases are you move across a group (top → bottom)
Consider as you move across a period, the protons increase generating stronger pull towards the electron. As you move across a group, although proton increases, the orbital shells also increase, increasing the atomic size
Ionic Size
All Cation Ions have a smaller ionic size compared to its parent atom
All Anion Ions have a greater ionic size compared to its parent atom
Since Cations lose electron, and Anions gain electron, Anions will occupy for electron shells thereby increasing ionic size.
Ionization Energy
Definition: The energy required to remove electrons from an atom/ion
Trend:
Increases as you move across the period (left → right)
Decrease as you move across the group (top → bottom)
As you move across the period, the amount of proton increases but the electrons stay on the same electron shell. Therefore there are more pull from the proton to the electron. Increasing the required energy. But as your move across the group, more electron occupy the further electron shell decreasing energy requirement.
Exception:
Group 2 > Group 13
Group 15 > Group 16
By the trend group 13 and group 16 should require more ionization energy than compared to group 2 and group 15 respectively. But because of the electron configuration, it is much easier to pull lone pairs than it is to pull paired electrons. Since Group 2 and Group 15 atoms have more paired electron, those group have greater ionization than Group 13 and 16.
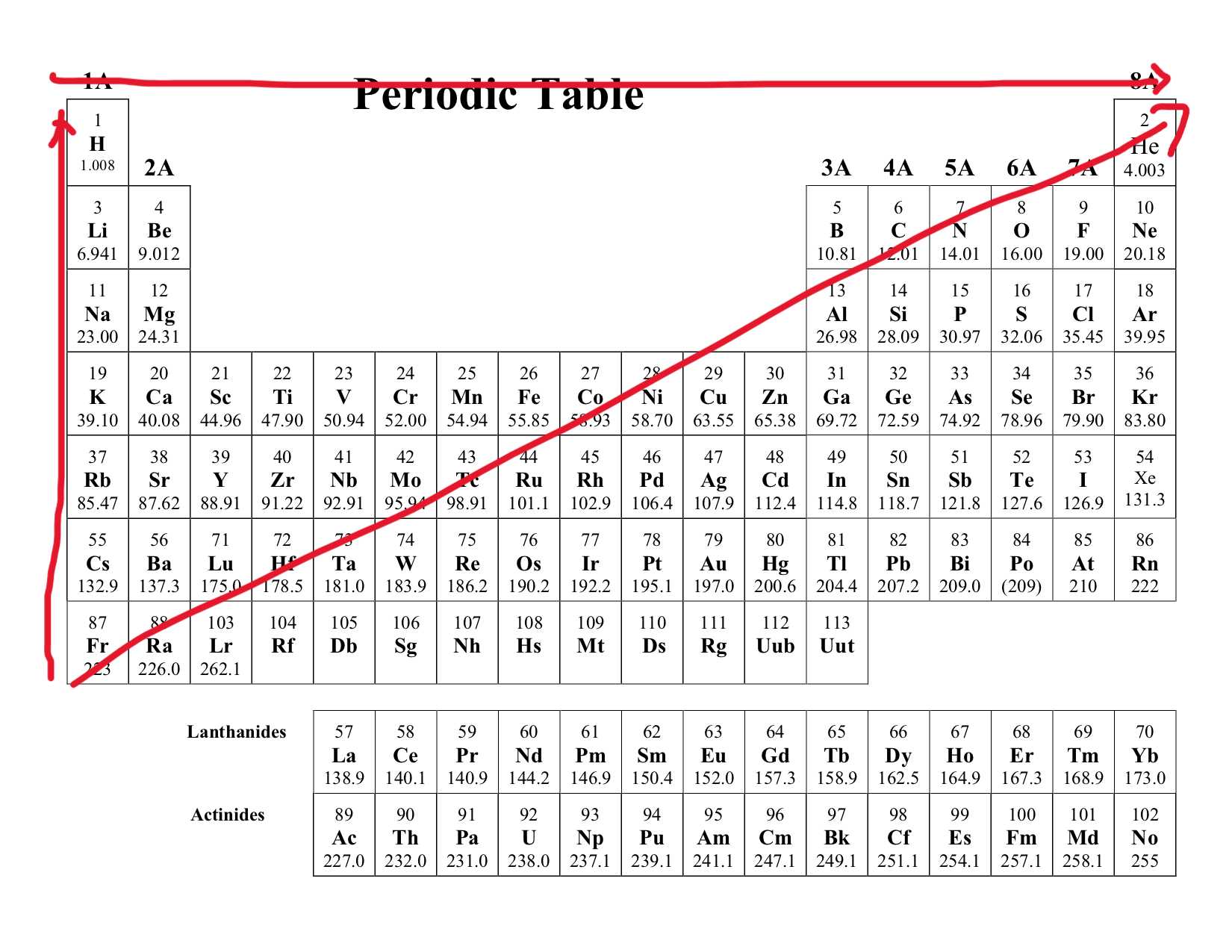
Electron Affinity
Definition: This describes the heat released when an atom/ion gains electron
Trend:
Increases as you move across the period (left → right)
Decreases as you move across the group (top → bottom)
As you move across the period the nuclear charge of the atom increases, making the atom more energetic to electrons. But as you move down the group the atomic radius increases decreasing proton attraction, and there is more electron shielding from the inner electrons.
Angular Node
This described the regions on the atomic orbitals that will not contain any electrons.
Determined by the Quantum Number L
Radial Node
This describes the region in the atomic orbital that will not contain any electrons.
Determined by the Quantum number n - L - 1
Electron Geometry:
2 Electron Group
Structure: Linear
Angle: 180
Electron Geometry:
3 Electron Group
Structure: Trigonal Planar
Angle: 120
Electron Geometry:
4 Electron Group
Structure: Tetrahedral
Angle: 109.5
Electron Geometry:
5 Electron Group
Structure: Trigonal Bipyramidal
Angle: (90, 120)
Electron Geometry:
6 Electron Group
Structure: Octahedral
Angle: 90
Molecular Geometry:
2 Electron Group
0 Lone Pair: Linear (180)
Molecular Geometry:
3 Electron Group
0 Lone Pair: Trigonal Planar (120)
1 Lone Pair: Bent
Molecular Geometry:
4 Electron Group
0 Lone Pair: Tetrahedral (109.5)
1 Lone Pair: Trigonal Pyramidal
2 Lone Pair: Bent
Molecular Geometry:
5 Electron Group
0 Lone Pair: Trigonal Bipyramidal (90, 120)
1 Lone Pair: Seesaw
2 Lone Pair: T-Shaped
3 Lone Pair: Linear
Molecular Geometry:
6 Electron Group
0 Lone Pair: Octahedral (90)
1 Lone Pair: Square Pyramidal
2 Lone Pair: Square Planar
3 Lone Pair: T-Shaped
4 Lone Pair: Linear
Formula:
Bond Order for Molecular Orbital Diagram
Bond Order = (Bond Electron - Antibonding Electron) / 2
Avogadro’s Number
6.022 × 1023
Arrhenius Acid
A substance that reacts in water to produce hydronium (H3O+) ion
Arrhenius Base
A substance that reacts in water to produce hydroxide (OH-) ion
Equation for Heat Energy
q = heat (J)
m = mass (g)
s = specific heat capacity
t = temperature (C)
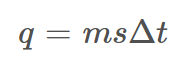
Speed of Light
c = 3.00 × 108 (m/s)
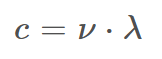
Equation for Speed of Light
c = 3.00 × 108 (m/s) (Speed of Light)
ν = frequency (s-1)
λ = wavelength (m)
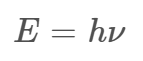
Equation for Energy of A Photon
E = Energy of Photon (J)
h = 6.626 × 10-34 (J * s) (Planck’s Constant)
v = frequency (s-1)
Rydberg’s Constant for Hydrogen
Rh = 1.09678 × 107 (m-1)
Rydberg’s Constant for Energy
Ry = 2.18 × 10-18 (J)
Equation for Rydberg’s Wavelength
λ = wavelength (m)
Rh = 1.09678 × 107 (m-1) Rydberg’s Constant for Hydrogen
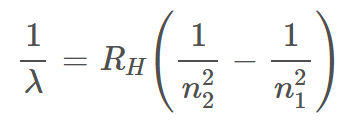
De Broglie’s Matter Wave Equation
λ = wavelength (m)
h = 6.626 × 10-34 (J * s) (Planck’s Constant)
m = mass (g)
v = speed (m/s)
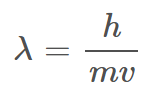
Rules for Assigning Oxidation Number
Oxidation number must add up to the total charge of the molecule, formula unit, or ion
All atoms of the free elements have an oxidation number of 0
Metals in 1A, 2A, and Al have an oxidation number of +1, +2, and +3, respectively
H and F in a compound have an oxidation number of +1 and -1, respectively
Oxygen has an oxidation number of -2
Group 7A has an oxidation number of -1
Group 6A has an oxidation number of -2
Group 5A has an oxidation number of -3
Specific Heat Capacity of Water
4.18 J/g C