Orgo II - Unit 5 - Alcohols, Diols, Thiols
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Aldehyde + H2/Metal (Reduction)
Primary Alcohol
Ketone + H2/Metal (Reduction)
Secondary Alcohol
Aldehyde + NaBH4 +water/methanol/ethanol (Reduction)
Primary Alcohol
Ketone + NaBH4 +water/methanol/ethanol (Reduction)
Secondary Alcohol
Use NaBD4 in aldehyde/ketone reduction instead of NaBH4
same concept
add D onto OH carbon instead of H (when breaking double bond)
Aldehyde + LiAlH4 +diethyl ether + H2O (Reduction)
Primary Alcohol
Ketone + LiAlH4 +diethyl ether + H2O (Reduction)
Secondary Alcohol
Carboxylic Acid + LiAlH4 +diethyl ether + H2O (Reduction)
Primary Alcohol
RMgX + Epoxide + diethyl ether + H3O
Primary Alcohol
Epoxide to Alcohol
Least crowded C attacked (pri preferred)
Dial
two aldehydes present
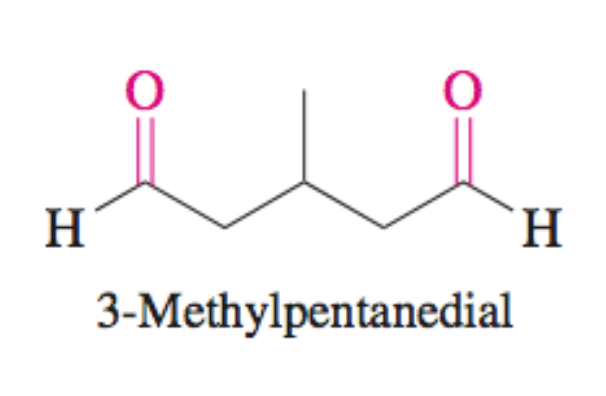
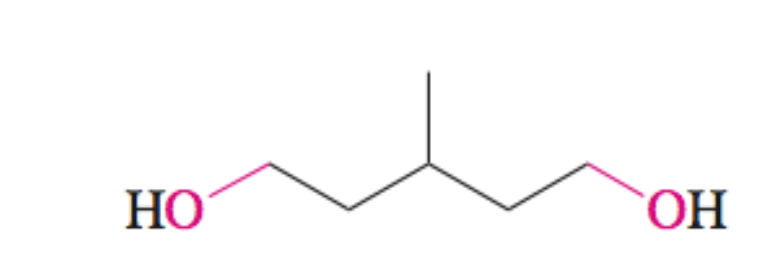
Dial + H2 + Ni
Forms diol
OH group at each aldehyde
Alkene + [OsO4 + (CH3)3COOH + OH + tert-butyl alcohol]
Vicinal Diol
Syn addition (OH groups are either both dashes or both wedges)
Only —— alcohols can be converted to ethers. Why?
Primary!
Secondary/Tertiary alcohols would undergo elimination
Primary Alcohol + H+/heat (or H2SO4/heat)
Ether
Diol + H2SO4/heat
Cyclic ether
Alcohol + Carboxylic Acid (+ H2SO4 for acidic conditions)
Ester
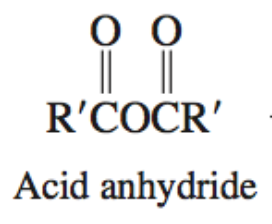
Alcohol + Acid Anhydride
Ester + COOH
Primary Alcohol + Cr2O7 + H2SO4 + H2O (Strong Ox Agent)
Carboxylic Acid
Primary Alcohol + PCC/PDC + CH2Cl2 (Weak Ox Agent)
Aldehyde
Alcohol + Chromic Acid
Alkyl hydrogen chromate
Alkyl hydrogen chromate + Water
Aldehyde or Ketone
Diol + Periodic Acid (HIO4)
Aldehyde or Ketone
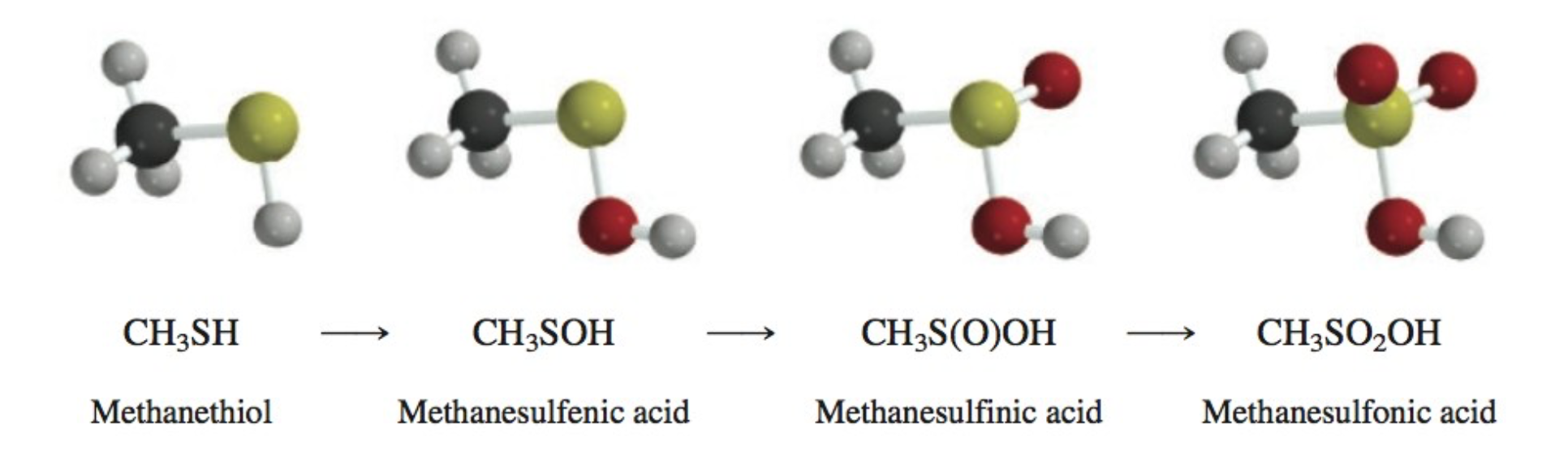
Thiols
Contain SH group
Bad odor
very acidic (more acidic than alcohols)
Oxidation of Thiols
Sulfoxides produced
Sulfur is a nucleophile with oxygen
Oxidation of Thiols Order
SH —> SOH —> S(O)OH —> SO2OH