Atomic Structure
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Atom
Smallest part of matter

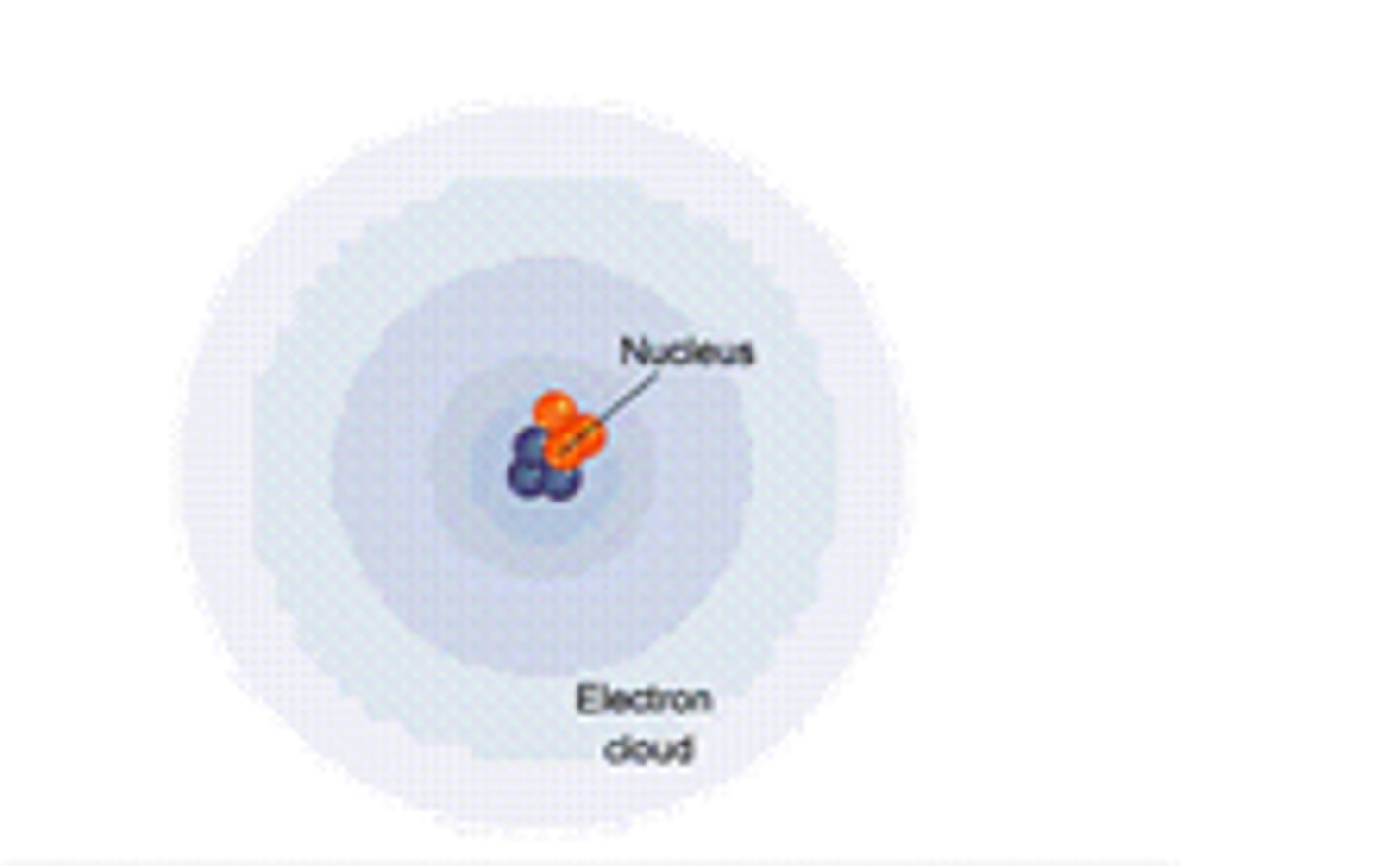
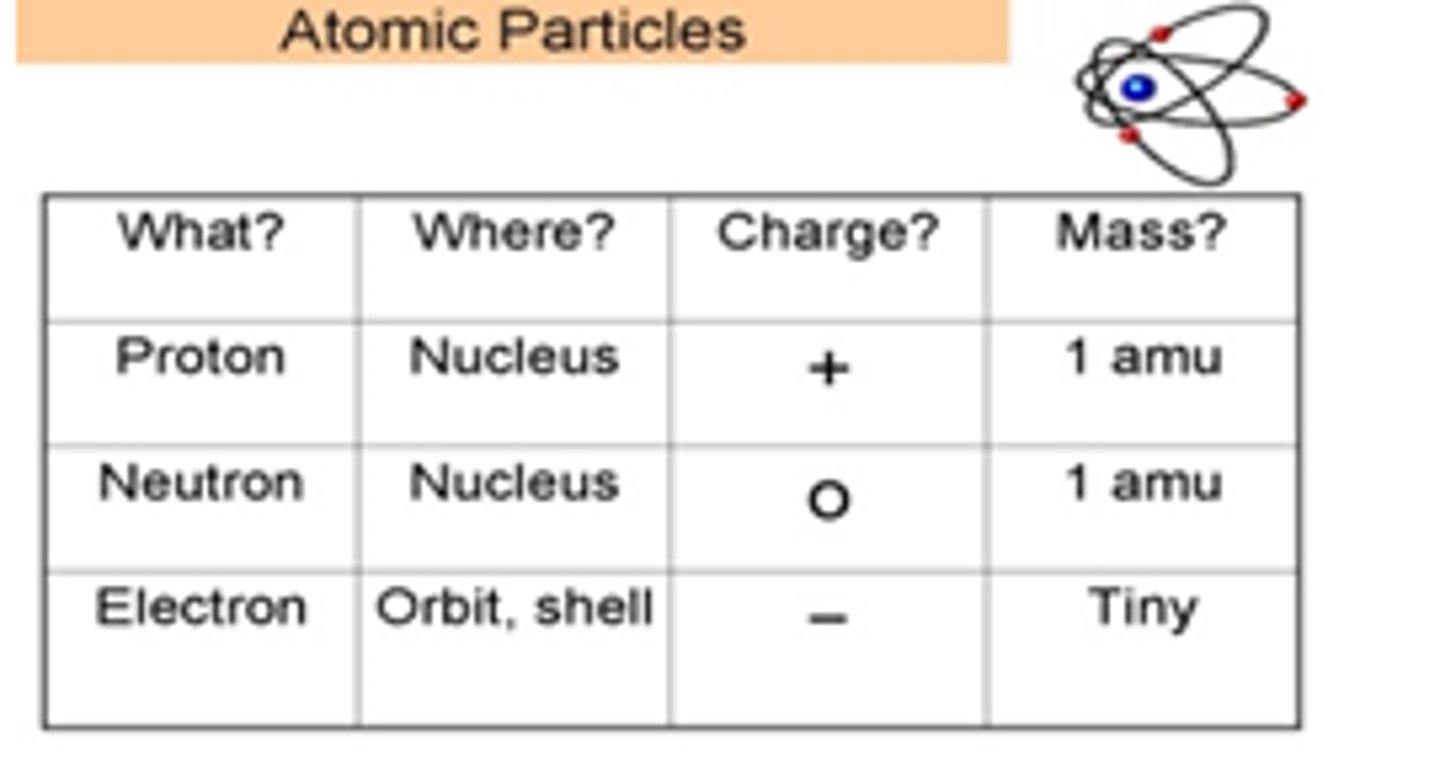
Nucleus
has a positive charge; center of the atom; contains protons and neutrons
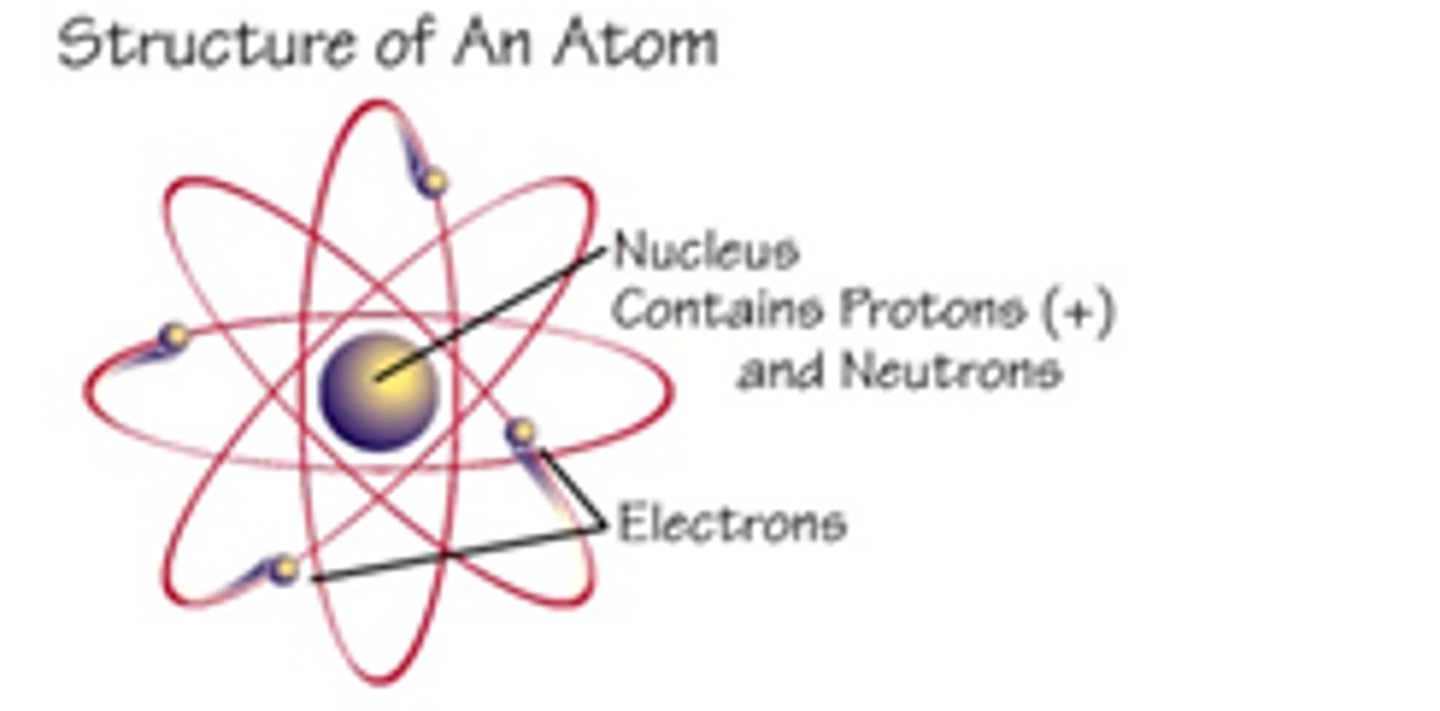
Proton
Positively charged subatomic particle. Found in the nucleus of the atom.
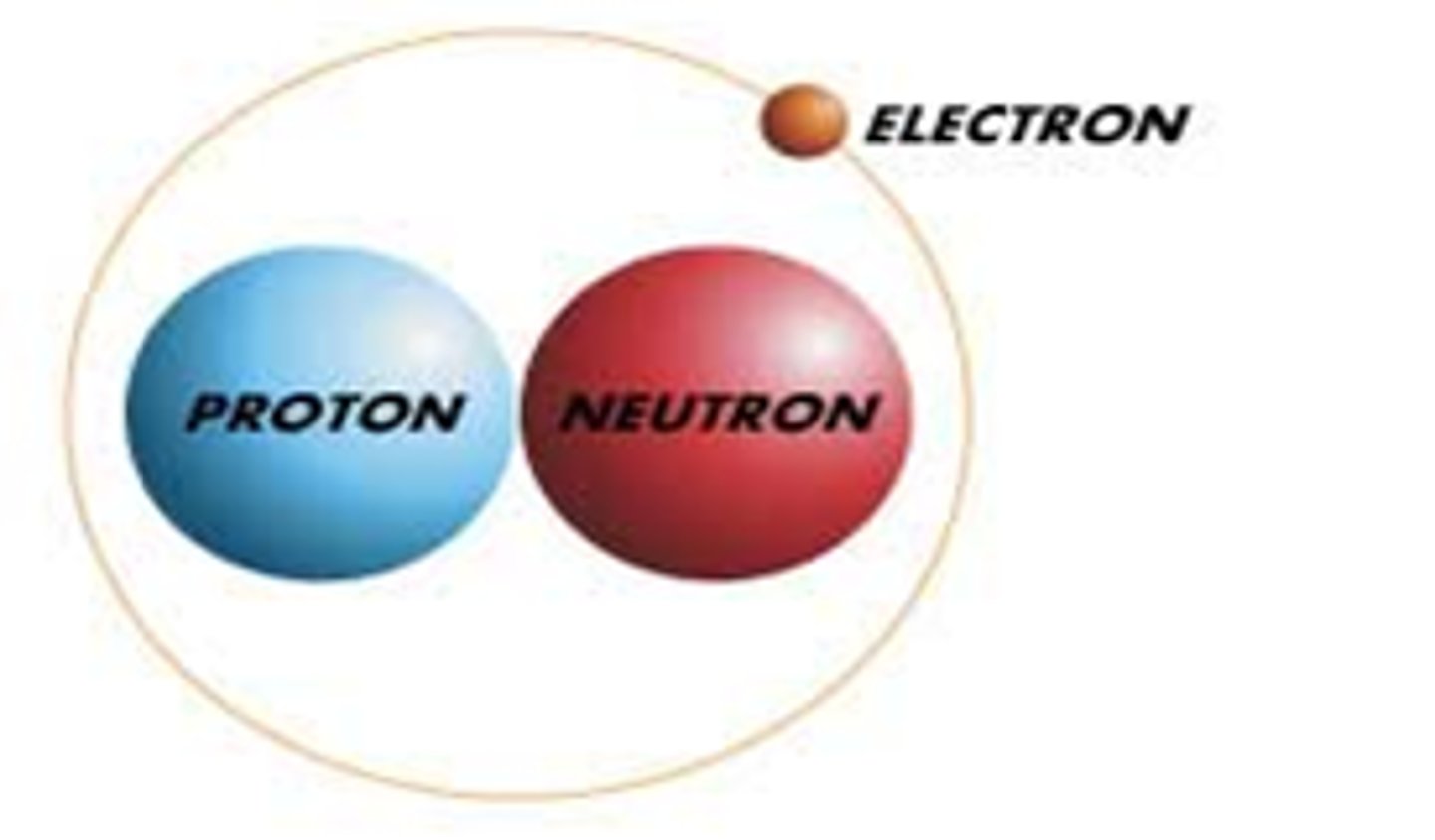
Electron
Negatively charged subatomic particle. Found outside of the nucleus in energy levels. Smallest particle...
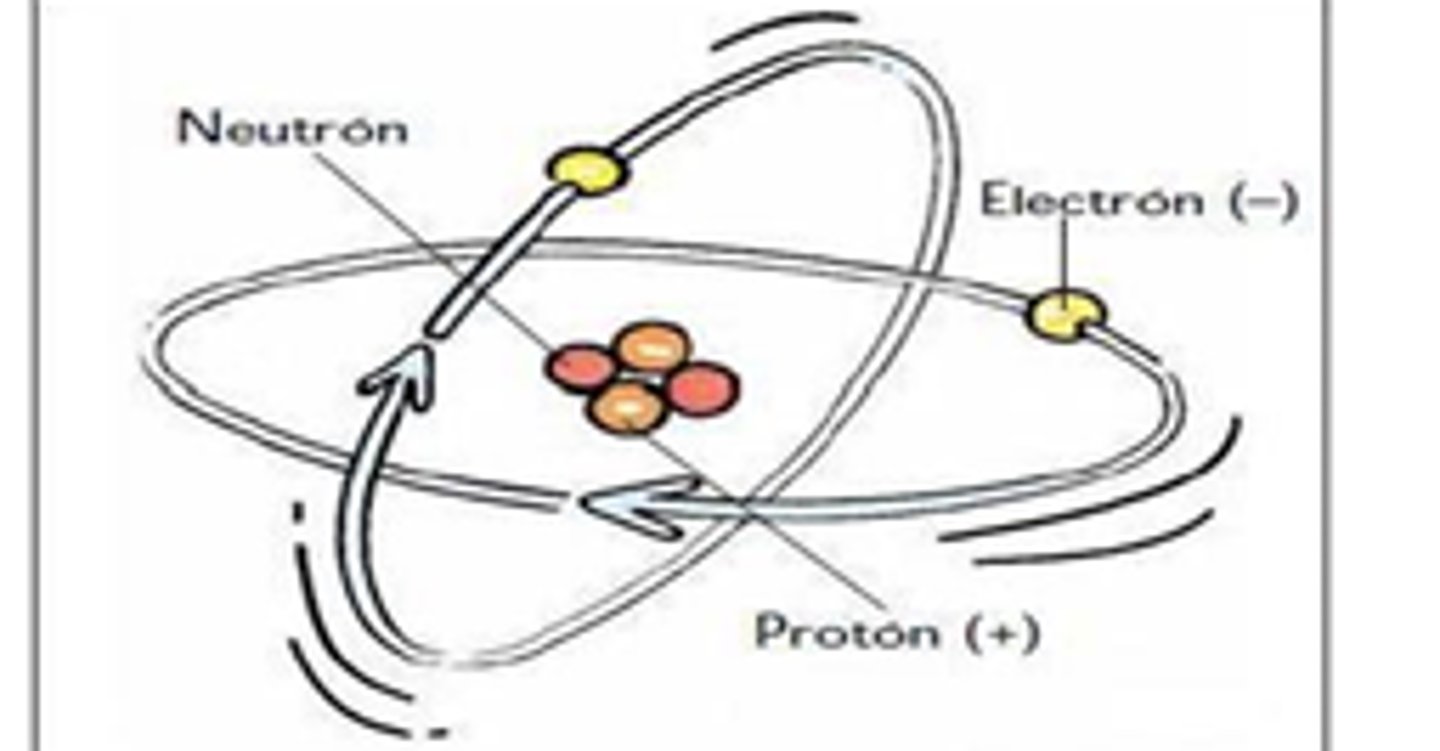
Neutron
Neutrally charged subatomic particle. Found inside the nucleus of the atom.
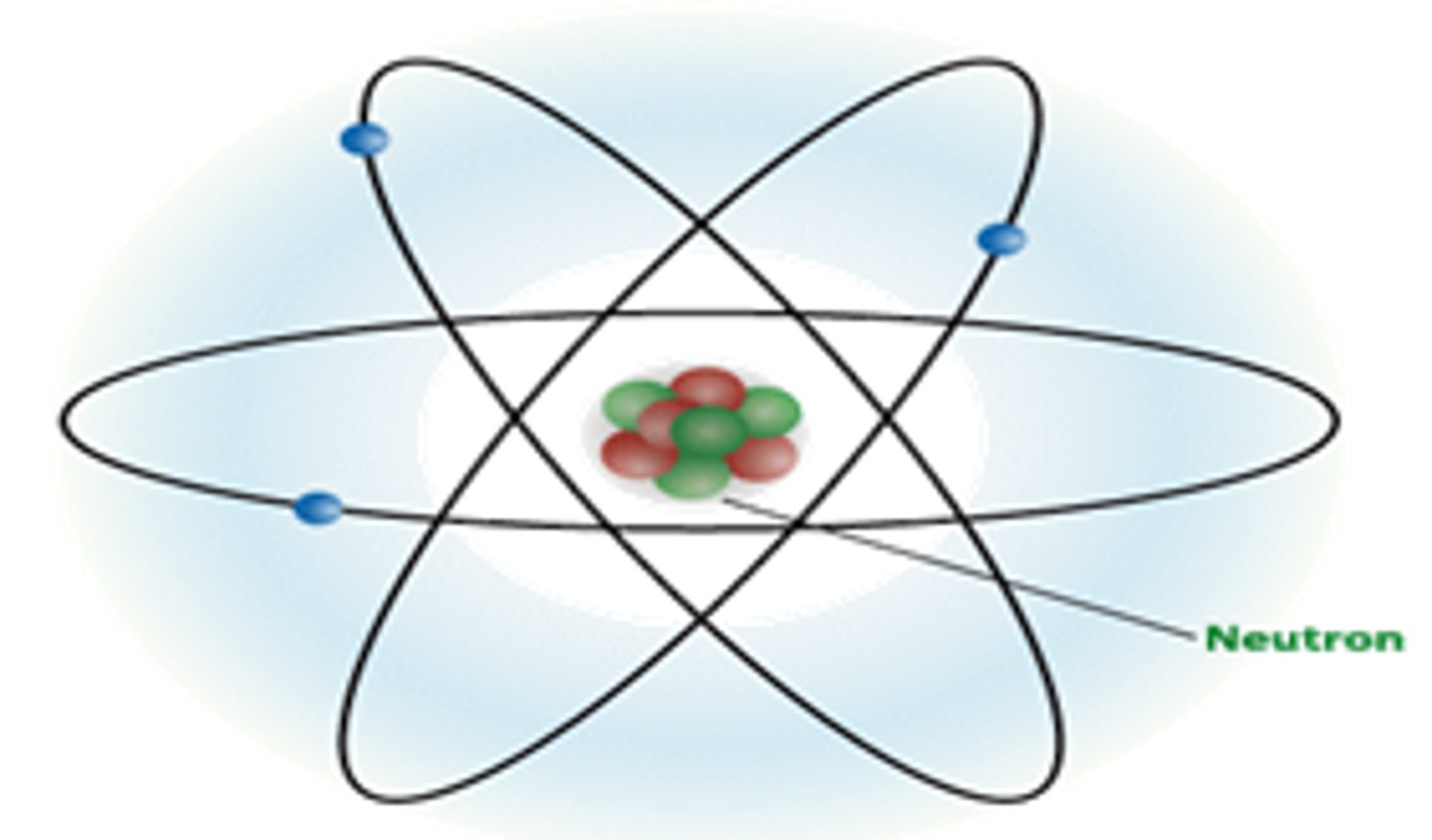
Energy Cloud / Energy Level
Space outside the nucleus where electrons orbit.
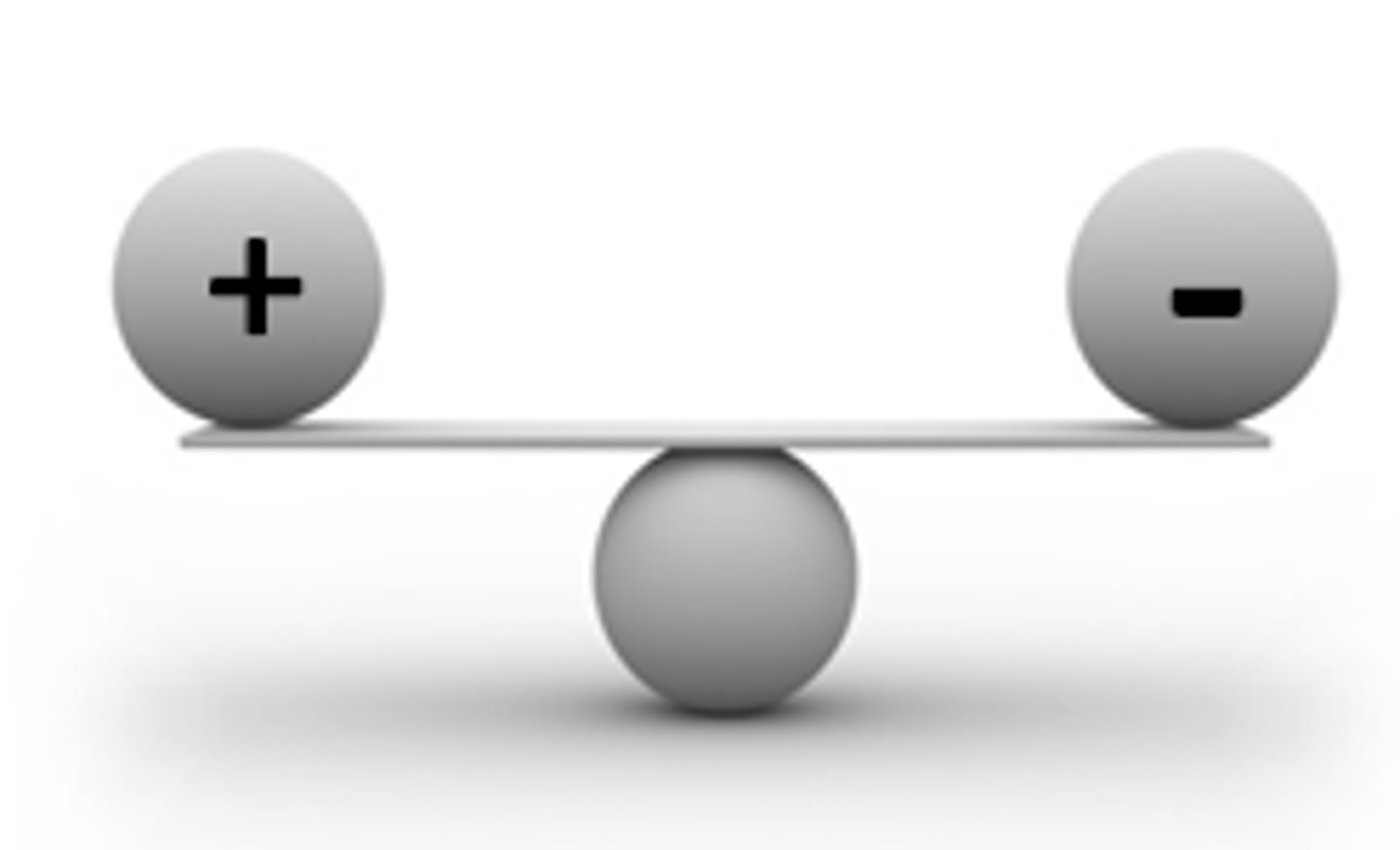
Neutral
an atom that has an equal number of protons and electrons
Valence Electron
The electrons in the outer most level. Used in bonding with other atoms.

Subatomic Particles
The small parts that make up the atom. Protons, electrons, and neutrons.
Atomic Number
Number of protons in the nucleus of an atom.
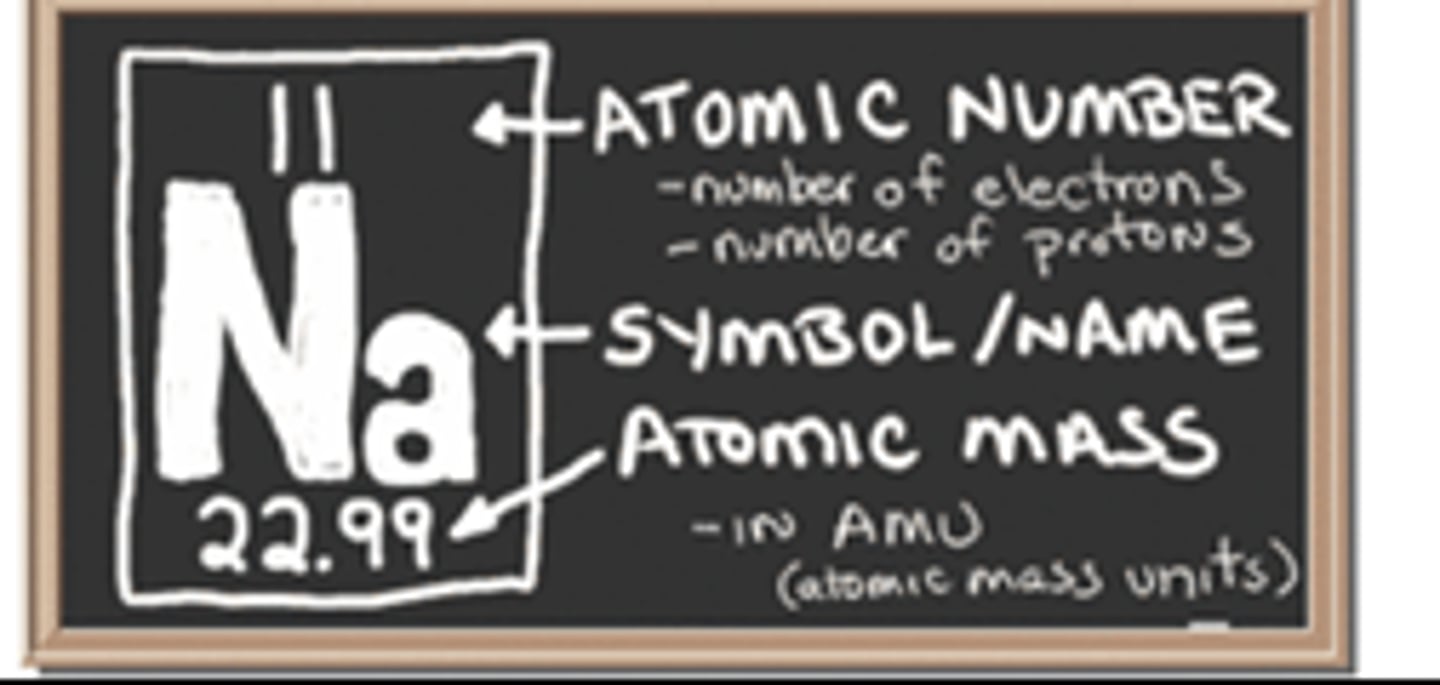
Atomic Mass
Number of protons and neutrons in an atom.
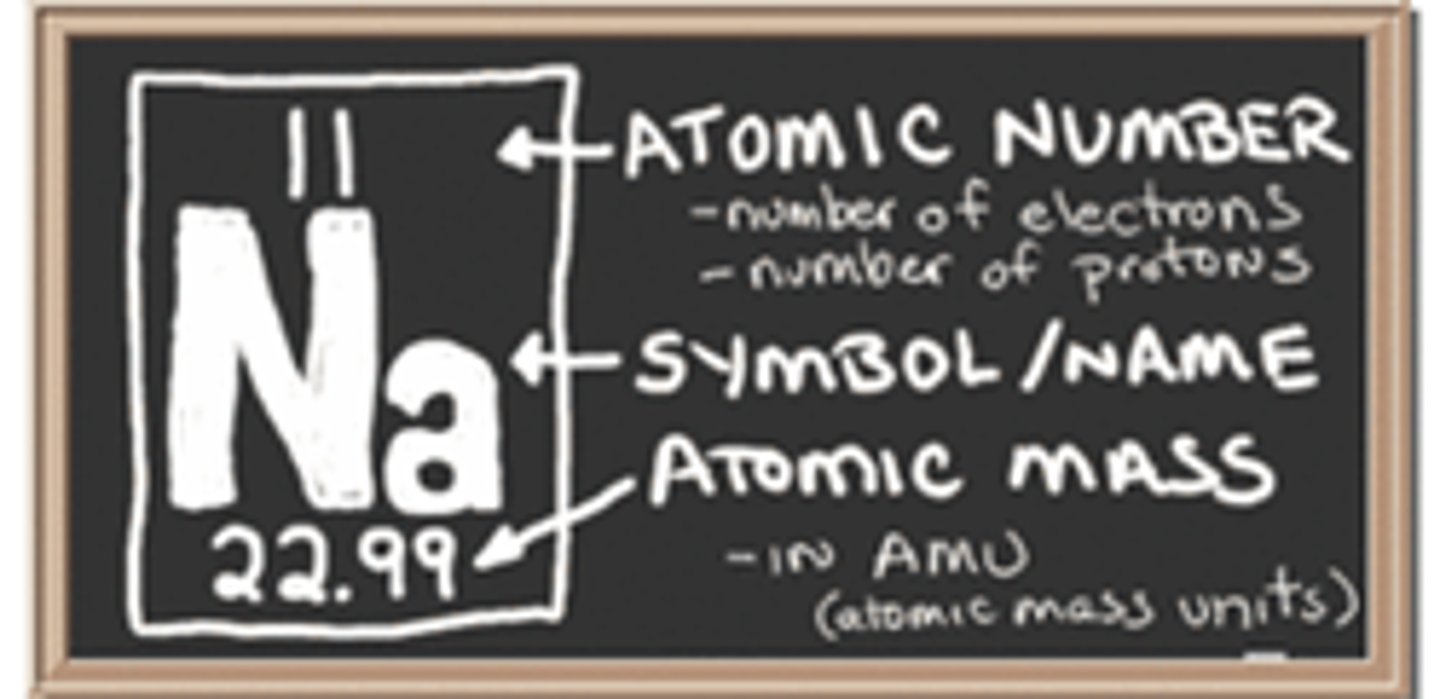
Atomic Mass Unit
A unit of mass that describes the mass of an atom or molecule.

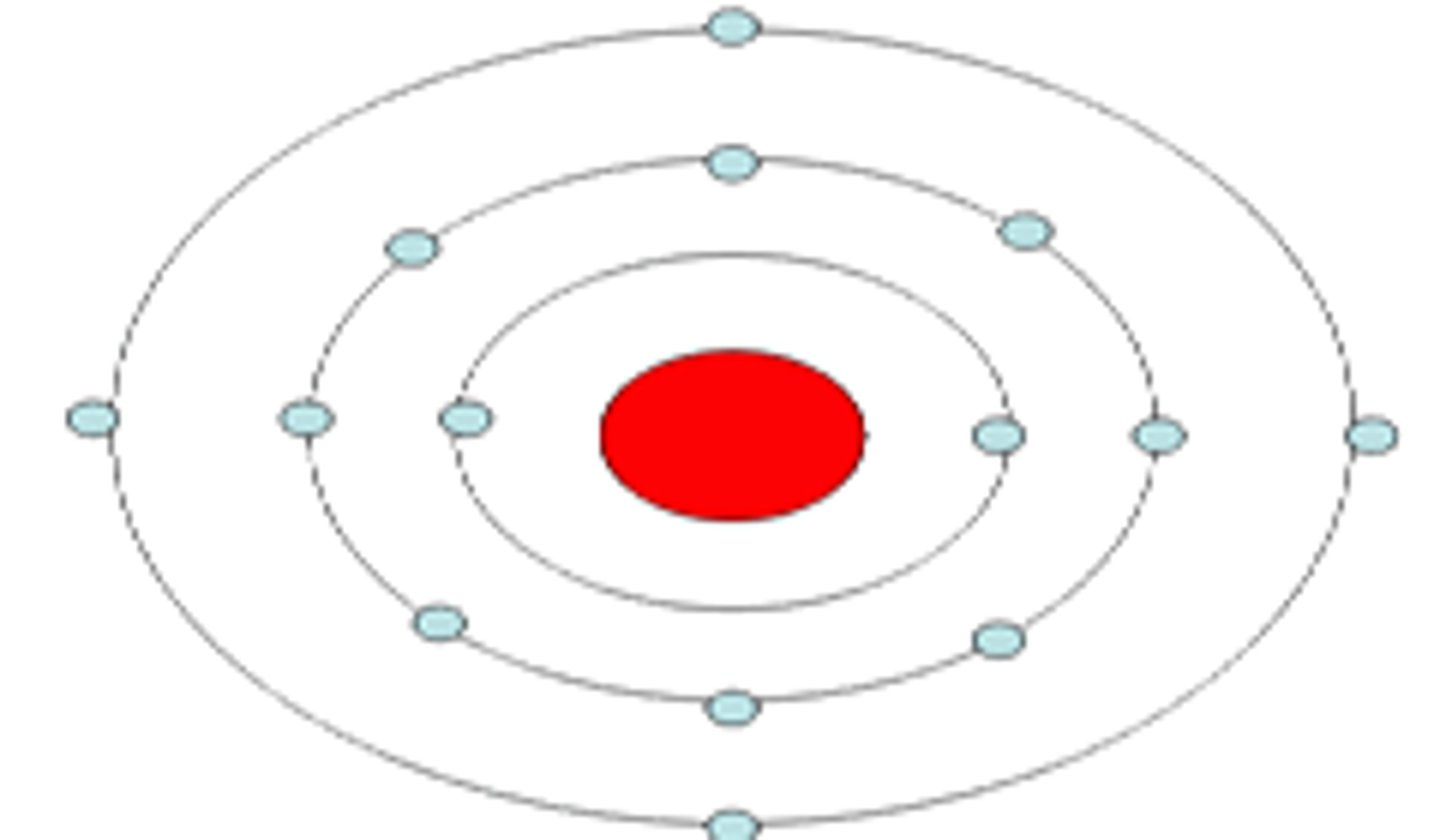
Bohr Model
model of the atom in which electrons move rapidly around the nucleus in paths called orbits
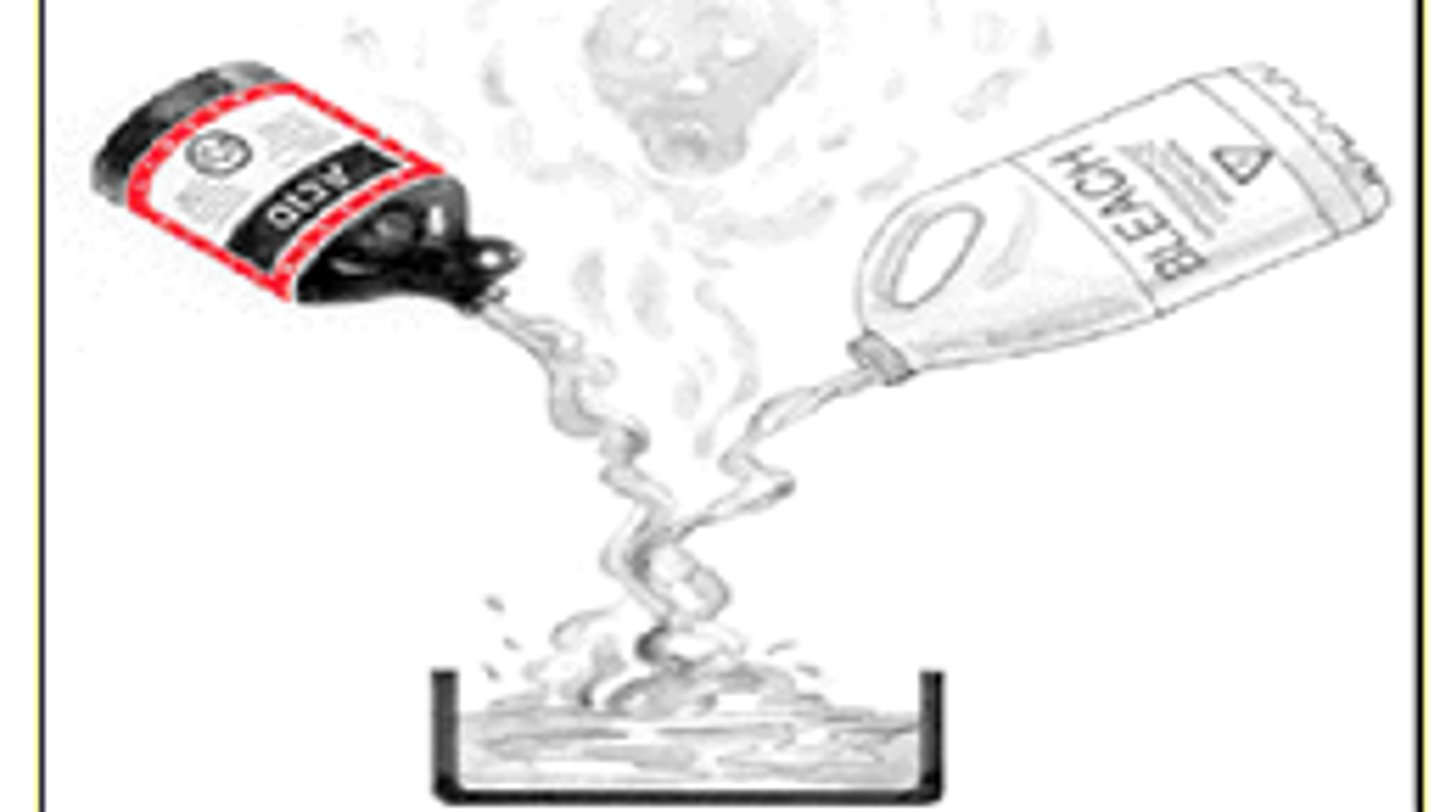
Reactivity
How readily a substance combines chemically with other substances.
Physical Property
A characteristic of a pure substance that can be observed without changing it into another substance.