Exp 4: Reductive Amination
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is a heterocycle?
a molecule that contains two different rings
any cyclic ring structure
a cyclic ring structure that contains only carbons and hydrogens
a cyclic ring structure that contains any element(s) outside of carbons and hydrogens
a non-cyclic molecule that becomes a cyclic molecule
a cyclic ring structure that contains any element(s) outside of carbons and hydrogens
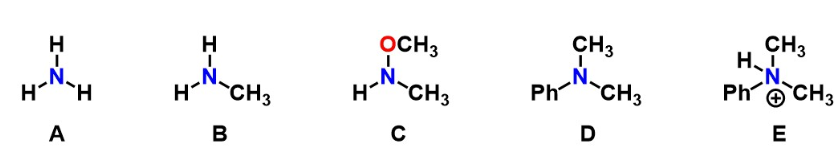
Which of the follow molecules represent an unsubstituted amine(s)?
A
Which of the following are true regarding the mechanism of imine formation?
The aldehyde attacks the amine
the amine attacks the carbonyl
formation of the hemiaminal is reversible
formation of the imine is reversible
the hemiaminal is more stable than the starting aldehyde and amine
the amine attacks the carbonyl
formation of the hemiaminal is reversible
formation of the imine is reversible
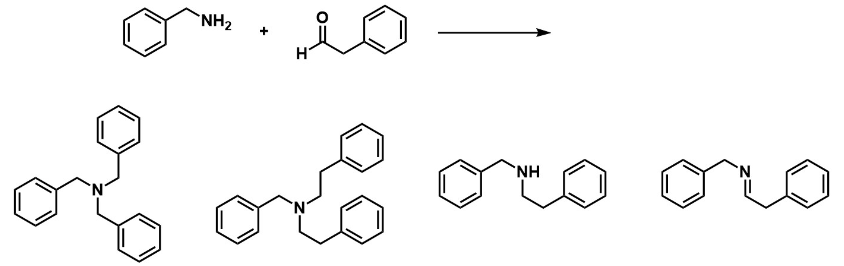
Predict the product for the following reaction:
D
The synthesis of compound X requires 2 steps. The first step was accomplished in 80% yield and the second step in 90% yield. What is the overall yield of the 2-step synthesis?
80%
90%
81%
64%
72%
72%
What is the purpose of mixing the 2 solids with a stirring rod?
to prepare the substances to react at a later stage
to purify the two solids
to recrystallize the two solids
to induce a chemical reaction between the two solids
it doesn't accomplish anything
to induce a chemical reaction between the two solids
What can happen if too much hexanes is used in the recrystallization?
the recrystallization yield can be low or even no crystals may form
hexanes is a non-polar solvent so there is no consequence for using too much
hexanes is a polar solvent so it can absorb water and complicate the recrystallization process
the yield will be >100%
the product may decompose
the recrystallization yield can be low or even no crystals may form
What is the most important piece of information that we can obtain from the IR spectrum at this step?
product formation can be distinguished by looking at the C-H stretches between 2850-3150 cm-1
product formation can be distinguished by looking for a new C=O stretch at approximately 1700 cm-1
product formation can be distinguished by the presence of an O-H stretch between 3200-3400 cm-1
product formation can be distinguished by looking for new C=C stretches at approximately 1600-1650 cm-1
product formation can be distinguished by the disappearance of the C=O stretch at approximately 1700 cm-1
product formation can be distinguished by the disappearance of the C=O stretch at approximately 1700 cm-1
Is p-toluidine or o-vanillin expected to be more polar?
Is p-toluidine or o-vanillin expected to be more polar?