Group Chemistry - Group 7 (halogens)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Understand reasons for the trend in melting and boiling temperatures
simple covalent diatomic molecules
weak London forces between each molecule
as you go down the group, the number of electrons per molecule increases
so the strength of the london forces increases
therefore more energy is required to overcome the stronger intermolecular london forces
and the mpt / bpt increases
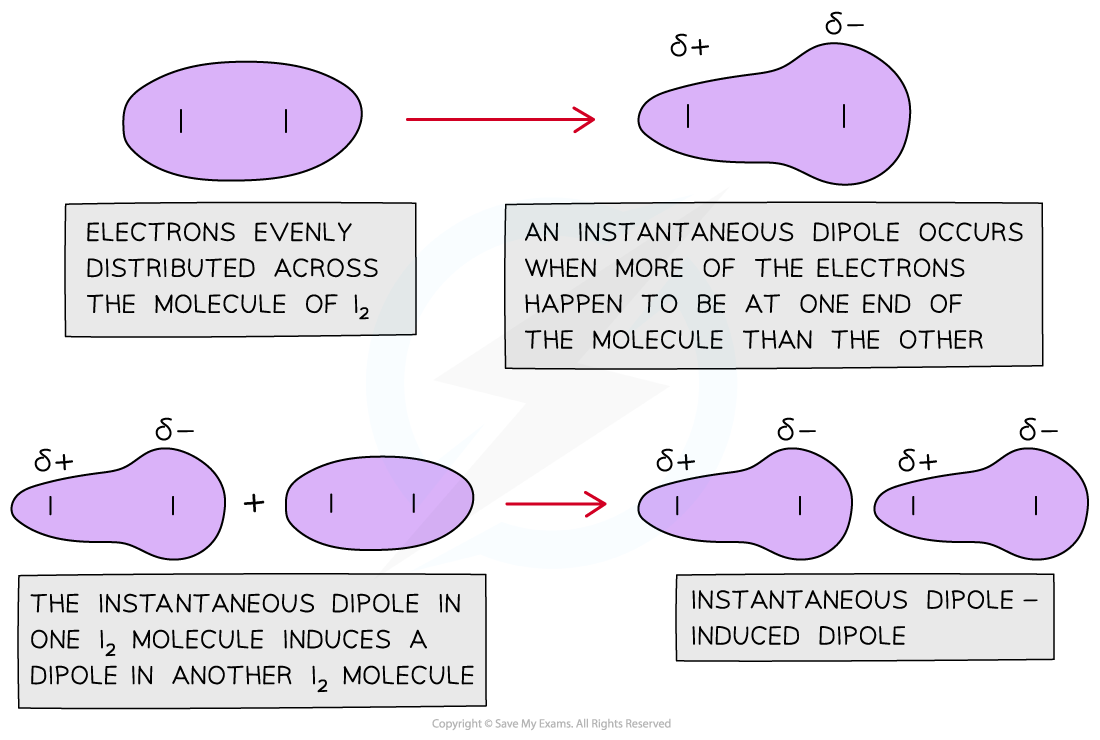
Understand reasons for the trends in physical state at room temperature
as mpt / bpt increase down the group
colour gets darker down the group
volatility decreases down the group
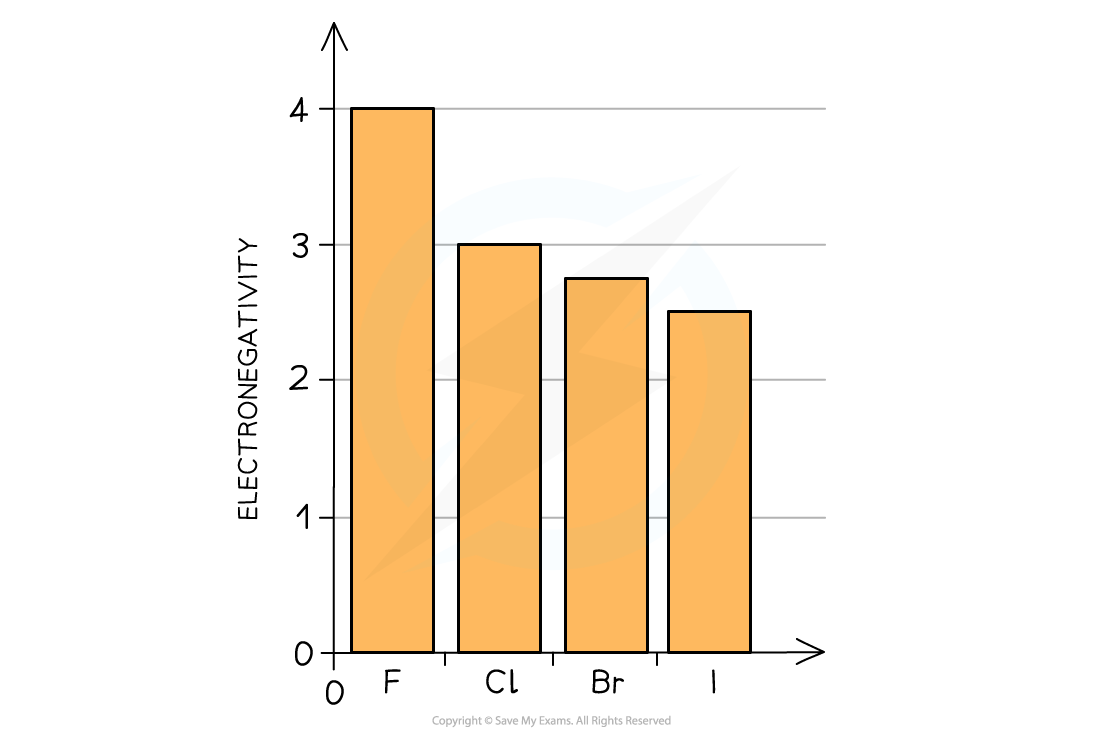
Understand reasons for the trends in electronegativity
Electronegativity = the ability of an atom to attract a bonding pair of electrons in a covalent bond
Going down the group:
atomic radius increase
incoming electron experiences greater amount of shielding
so halogen’s ability to accept an electron (oxidising power) decreases down the group
therefore electronegativity decreases down the group
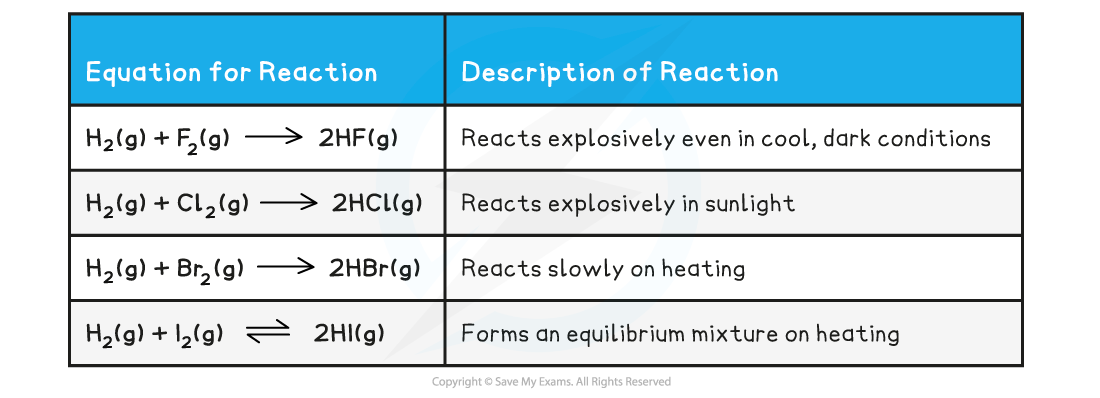
Understand reasons for the trend in reactivity of group 7 elements down the group
Down group 7:
atoms are larger
outer electrons further away from positive nucleus
larger atoms find it more difficult to attract incoming electrons needed to form 1- anion
therefore reactivity decreases
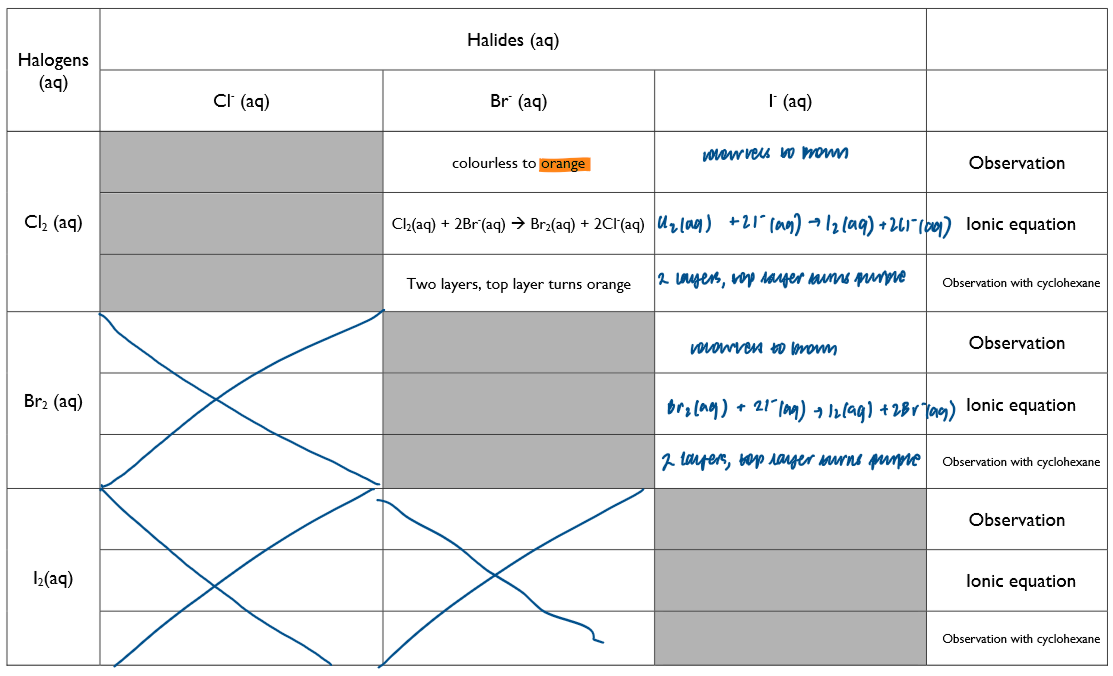
Understand the trend in reactivity of group 7 elements in terms of the redox reactions of Cl2, Br2 and I2 with halide ions in aqueous solution, followed by the addition of an organic solvent
Place about 2cm³ of the halide solution in a test tube and then add a similar depth of the halogen solution. Place a bung in the test tube and shake.
look carefully for colour changes, bearing in mind that if no reaction takes place the halogen solution will have been diluted and become paler in colour
add 2cm³ of cyclohexane. Place the bung in the test tube and shake. Make a note of the colour.
Adding cyclohexane should make it more obvious if a displacement reaction has taken place because the colour of a halogen in an organic solvent is the same as the colour of its vapour.
Understand, in terms of changes in oxidation number, the following reactions of the halogens:
Oxidation reactions with group 1 and 2 metals
with metals such as magnesium, the halogens react to form ionic metal halides.
Br2 (l) + Mg (s) → MgBr2 (s)
Mg → Mg2+ + 2e- (oxidation)
Br2 + 2e- → 2Br- (reduction)
Understand, in terms of changes in oxidation number, the following reactions of the halogens:
The disproportionation reaction of chlorine water and the use of chorine in water treatment
Test for chlorine gas: with damp blue litmus paper
Result: the blue litmus paper initially turns red and then gets bleached
Cl2 (g) + H2O (l) → HCl (aq) + HOCl (aq)
where HCl is hydrochloric acid and HOCl is chloric (I) acid
At very low concentrations, chlorine is used to disinfect tap water. It forms chloric acid when it reacts with water. Chloric (I) acid is a powerful oxidising agent and a weak acid. It is an effective disinfectant because the molecules can pass through the cell walls of bacteria. Once inside the bacterium, the HClO molecules break the cell open and kill the organism by oxidising and chlorinating molecules which make up its structure.
Swimming pools can be sterilised with much higher concentrations of chlorine compounds which react to form chloric (I) acid when they dissolve in water.
Understand, in terms of changes in oxidation number, the following reactions of the halogens:
The disproportionation reaction of chlorine with cold, dilute aqueous sodium hydroxide to form bleach
Cl2 (g) + 2NaOH (aq) → NaClO (aq) + NaCl (aq) + H2O (l)
Cl2 (g) + 2OH- (aq) → ClO- (aq) + Cl- (aq) + H2O (l)
this is a disproportionation reaction
where NaClO is bleach
Understand, in terms of changes in oxidation number, the following reactions of the halogens:
The disproportionation reaction of chlorine with hot alkali
If sodium hydroxide and chlorine are reacted at about 80 degrees Celsius, then the products are sodium chlorate (V), NaClO3, as well as sodium chloride and water.
3Cl2 (g) + 6NaOH (aq) → NaClO3 (aq) + 5NaCl (aq) + 3H2O (l)
3Cl2 (g) + 6OH- (aq) → ClO3- (aq) + 5Cl- (aq) + 3H2O (l)
This is a disproportionation reaction
Understand the following reactions:
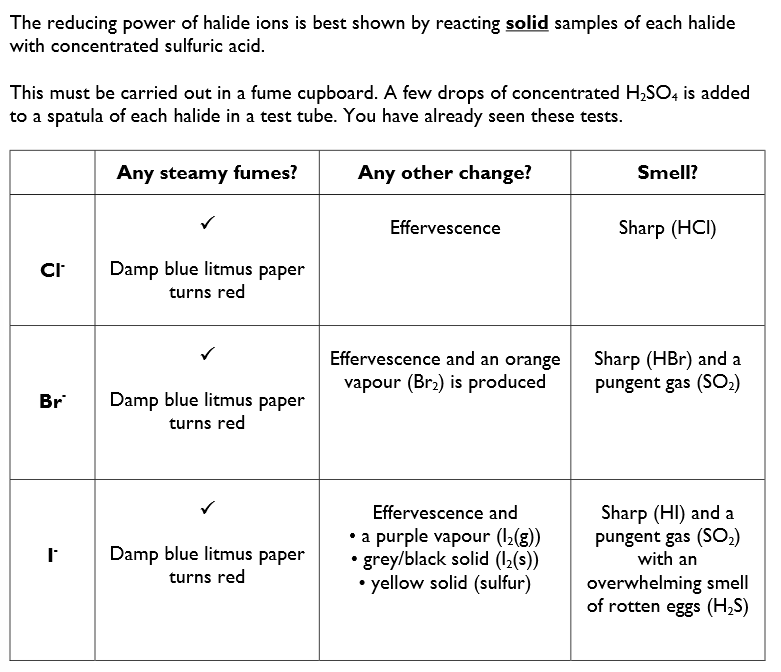
Solid group 1 halides with concentrated sulphuric acid, to illustrate the trend in reducing ability of the hydrogen halides
Chloride, Cl-
With potassium chloride the only reaction that occurs is:
KCl (s) + H2SO4 (aq) → HCl (g) + KHSO4 (aq)
No change in oxidation number, therefore reaction is not a redox reaction.
Bromide, Br-
A similar reaction occurs with all the potassium halide. Write an equation showing the formation of hydrogen bromide.
KBr (s) + H2SO4 (aq) → K2SO4 (aq) + HBr (g)
With potassium bromide a redox reaction also occurs, as well as the above reaction. The Br- ions are oxidised to Br2 and the SO4 2- ions are reduced to SO2.
2Br- + 4H+ + SO4 2- → Br2 + SO2 + 2H2O
Bromide ions are better reducing agents than chlorine ions.
Iodide, I-
With potassium iodide other redox reactions also occur (as well as the reactions above). With KI, the I- ions are oxidised to I2 and the SO4 2- ions are reduced to S and H2S.
6I- + SO4 2- + 8H+ → 3I2 + S + 4H2O
SO4 2- + 10H+ + 8I- → 4I2 + H2S + 4H2O
When Br- ions react with concentrated H2SO4, sulfur is reduced from +6 to +4.
In a similar reaction using I- ions, the sulfur is reduced + 6 to -2.
Therefore I- halide has the stronger reducing action on concentrated H2SO4, as it shows the greatest extent of reduction.
I- > Br- > Cl-
A reducing agent loses electrons in a reaction.
The I- ions has the biggest ionic radius and the most amount of shielding so the attraction between the positive nucleus and outermost e- is weaker so the e- is more easily lost.
Understand the following reactions:
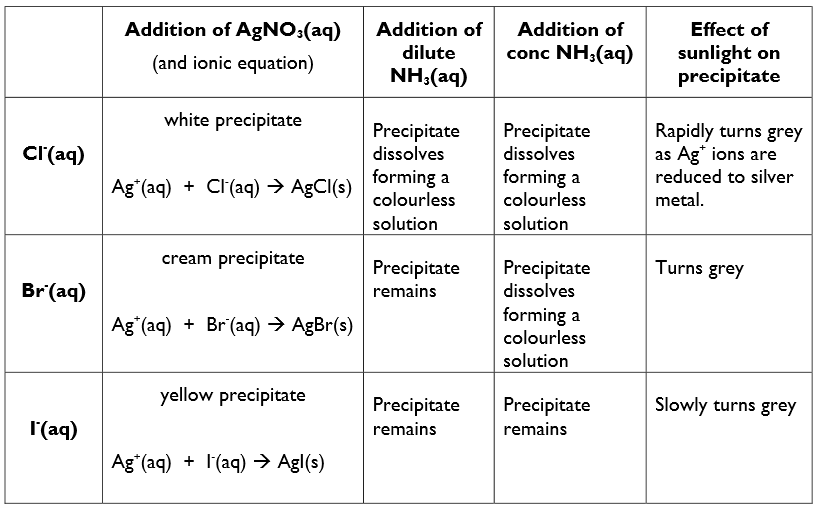
Precipitation reactions of the aqueous anions Cl-, Br- and I- with aqueous silver nitrate solution, followed by aqueous ammonia solution
Add a few drops of nitric acid (aq) to the solution being tested
This removes any other ions that could give a precipitate with silver nitrate (aq)
Add silver nitrate (aq) dropwise to the solution being tested
this produces a ppt for Cl-, Br- and I- ions
Add ammonia (aq) (dilute + conc) to the ppt
this is used to see if the ppt re-dissolves to help confirm their identity.
Understand the following reactions:
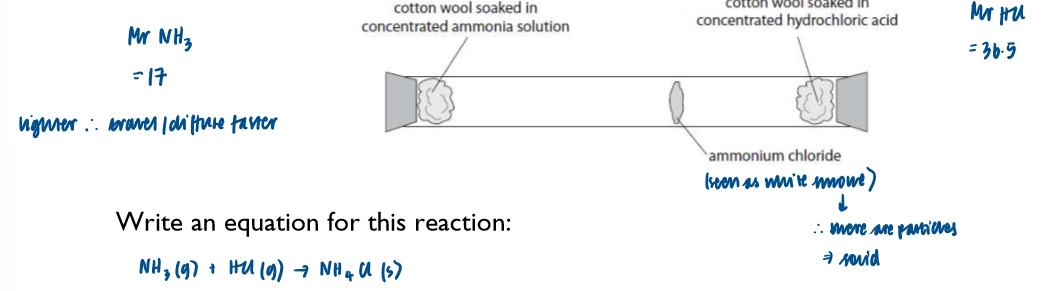
Hydrogen halides with ammonia and with water (to produce acids)
Reaction with ammonia:
if a hydrogen halide gas and ammonia gas are mixed, a white cloud of the ammonium halide appears
N.B. This is NOT a gas, it is tiny solid airborne particles which eventually settle as dust.
NH3 (g) + HCl (g) → NH4Cl (s)
This can be used as a test for a hydrogen halide - but it will not show which halogen is present
Be able to make predictions about fluorine and astatine and their compounds, in terms of knowledge of trends in halogen chemistry
fluorine - pale yellow gas
astatine - dark black solid
The reaction of halogens with non-metals
with non-metals, such as phosphorus, halogens react to form covalent halides
¼ P4 (s) + 3/2 Cl2 (g) → PCl3 (l)
The Cl changes from 0 to -1 therefore it is reduced and is acting as the oxidising agent
The P changes from 0 to +3 therefore it is oxidised and is acting as the reducing agent
Describe properties of the hydrogen halides:
all halogens form covalently bonded compounds with hydrogen
at room temp they are colourless gases that fume in moist air
all hydrogen halides are simple molecules therefore not much energy is needed to overcome the weak intermolecular forces
they are extremely soluble in water
- this is partly because they are polar molecules and water is a polar solvent
however it is mainly because in aqueous solution the molecules fully dissociate into ions, which then become hydrated, releasing lots of heat energy (very exothermic)
HX + (aq) → H+ (aq) + X- (aq)
the presence of H+ ions means that solutions of the hydrogen halides are acidic
the acidic character of hydrogen halides means they can react with bases, for example, ammonia and potassium or sodium hydroxides.
group 1 halides are ionic compounds
they are white crystalline solids, soluble in water giving colourless solutions