ionic and covalent bonds
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
naming ionic compounds
cation is named first as the element name
anion is named second, elements name changes to an -ide ending
criss cross method
only used for unequal charges
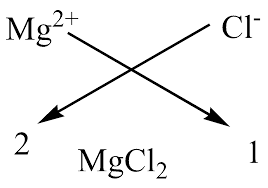
ionic compound
a metallic element loses electrons, an non metallic element gains electrons to form a cation/anion pair
ionic compounds are always….
metal and non meta;
the only way for metallic elements to satisfy the octet rule is…
ionic compounds
covalent compounds
two or more non- metals share electrons to satisfy the octet rule
covalent compounds are always…
two or more non metals
naming covalent compounds
central atom is named as element
other atoms change element name ending in - ide
apply numeric prefixes (never use ‘mono’ on first element)
covalent compound numeric prefixes
1- mono
2- di
3- tri
4- tetra
5- penta
6- hexa
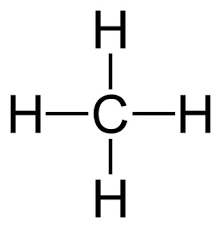
lewis dot structure
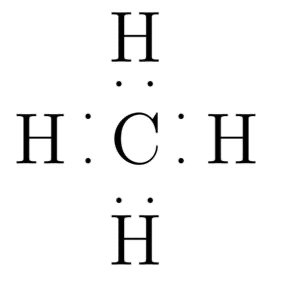
line bond structure