Orgo1 FunctionalGroups
1/37
Earn XP
Description and Tags
ATEEE :>
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Hydrocarbons
alkane,alkene, benzene, alkyne
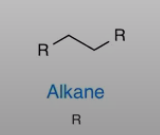
Alkane
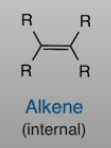
Alkene internal
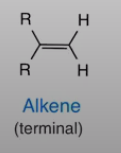
Alkene terminal
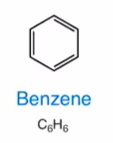
Benzene
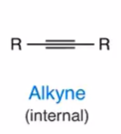
Alkyne (internal)
alkyne = triple bond
the y looks like 3 strokes, triple bond…
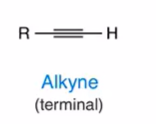
Alkyne terminal
our last hydrocarbon!
Alkyl Halide
X means F, Cl, Br, Iodine
halogen
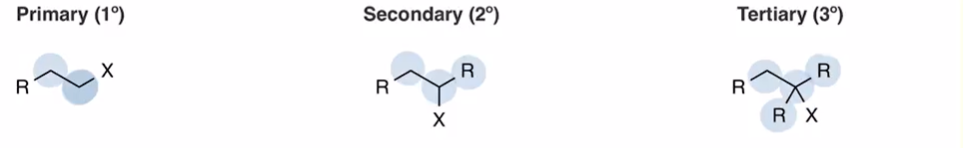
Primary (bonded to one other carbon) secondary and teritiary
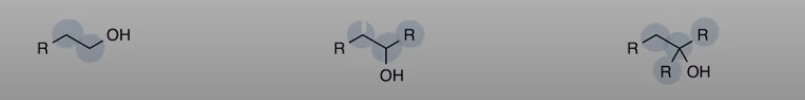
Alcohol
Carbon bonded to OH
primary, secondary and teritiary
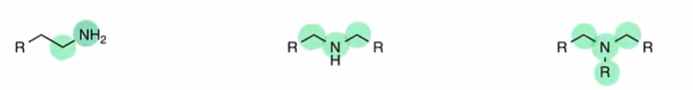
Amine
RNH2, R2NH, R3N all represent amine (R representing carbon chain)
classified by direct carbon attachments nitrogen is making
primary secondary and teritiary
Oxygen containing functional groups
alcohols, ether, epoxide
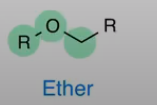
Ether
Oxyen directly bonded to 2 carbon chains
NEVER double bonded O (thats ester)
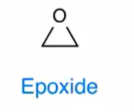
Epoxide
oxygen directly bonded to 2 carbon chains
greater reactivity than ether
Nitrogen containing
amine, nitrile, quaternary ammonium salt
Nitrile
carbon nitrogen triple bond
ex NaCN
or CH3CN

Quatenary ammonium salt
nitrogen atom bonded to 4 atoms giving nitrogen a pos formal charge
could interact with halogen

Sulfur containing functional groups
Thio = sulfur containing (or mercapto)
thiol, thioether, thioester, disulfide, sulfonium salt
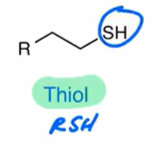
Thiol
RSH
thioether
sulfur directly bonded to carbon chains
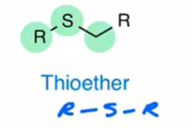
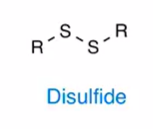
disulfide
2 sulfur bonded to each other
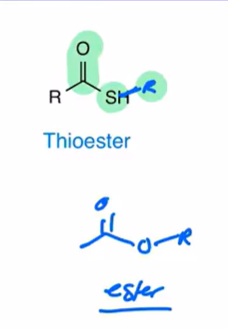
Thioester
sulfur attacched to alkyl group attached to carbonyl
Sulfonium salt
sulfur making bonds with 3 carbons, sulfur has pos formal charge sulfur counterbalanced by negative halogen

Carbonyl compounds
Carbonyl: carbon atom double bonded to oxygen atom
acyl chloride
acid anhydride
carboxylate
carboxylic acid
aldehyde
ketone
ester
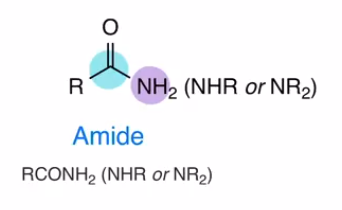
amide
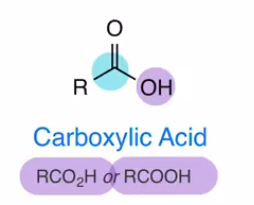
Carboxylic acid
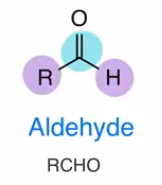
Aldehyde
like an acyl chloride, only there’s a hydrogen instead of chloride
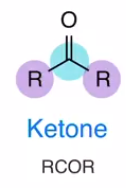
Ketone
Carbonyl carbon attached to 2 carbon groups
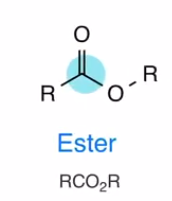
Ester
Amide
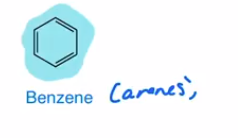
Aromatics (arenes)
cyclic, planar, every atom has sp2 hybridization state, 4n+2← # of pi electrons creating a pi cloud, when the pi cloud has a certain # of electrons it ends up being very stable
Benzene
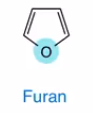
Furan
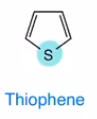
Thiophene
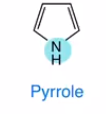
Pyrrole
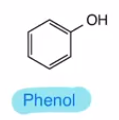
Phenol
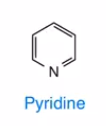
Pyridine
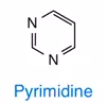
Pyrimidine
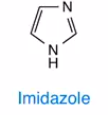
Imidazole
Benzene
Furan
Pyridine
THIS IS ALL YOU NEEDT O KNOW
have a nice day =)
Thiophene
Pyrrole
Phenol
Pyrimidine
Imidazole
HAHAHHA :<