PLASMA PROTEINS
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Plasma proteins
Plasma proteins are proteins present in blood plasma, the liquid component of blood. They play crucial roles in maintaining homeostasis, transporting substances, immune responses, and blood clotting.
General charecteristics of plasma proteins
They are synthesized in liver except immunoglobulin which is synthesised by plasma cells
Almost all plasma proteins are glycoproteins.
Plasma proteins are soluble in water, which allows them to remain suspended in the blood plasma and perform their functions efficiently.
Plasma proteins carry a negative charge at physiological pH (around 7.4). This charge helps to keep them in suspension in the plasma and prevents them from precipitating.
The concentration of plasma proteins in the blood is usually around 6-8 g/dL. Albumin is the most abundant, making up about 55-60% of the total protein concentration.
General functions of plasma proteins
Transport: Plasma proteins bind and carry various substances such as hormones, vitamins, minerals, drugs, and waste products throughout the body.
Osmotic Pressure: They maintain oncotic (colloid osmotic) pressure, which helps retain water in the blood vessels and prevents fluid from leaking into tissues (edema prevention).
Defense Mechanisms: Some plasma proteins, particularly immunoglobulins and complement proteins, play critical roles in the immune response against pathogens.
Blood Clotting: Proteins like fibrinogen are essential for the blood clotting process, helping to stop bleeding and heal wounds.
Regulation of Inflammation: Certain plasma proteins are involved in the regulation of inflammatory responses, helping the body respond to injury or infection.
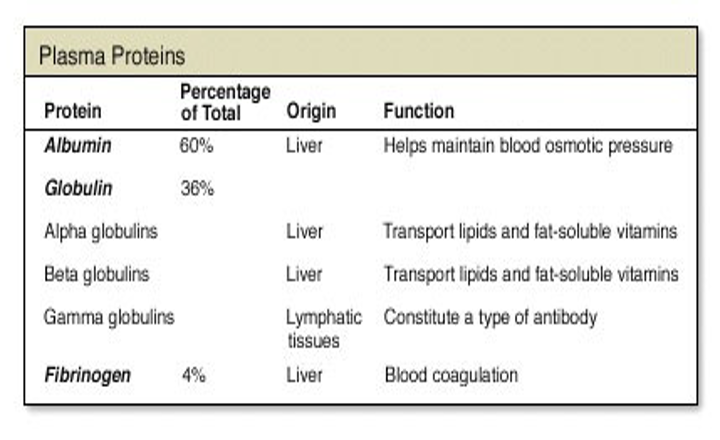
Major Plasma proteins
Albumin
Globulin (alpha,beta,gamma)
Fibrinogen
C-reactive protein
Transferin
Albumin
Structure:
Albumin is a small, single-chain protein with a molecular weight of about 66.5 kDa. It has a flexible structure with multiple binding sites for various substances.
Function:
Osmotic Pressure: Maintains colloid osmotic pressure, crucial for preventing fluid from leaking out of blood vessels into surrounding tissues.
Transport: Binds and carries a variety of substances including fatty acids, hormones, bilirubin, and drugs.
Buffering: Helps maintain pH balance in the blood by acting as a weak acid.
Clinical Significance:
Hypoalbuminemia: Low albumin levels can lead to edema, as seen in liver disease, nephrotic syndrome, and malnutrition.
Hyperalbuminemia: Rare and typically due to dehydration or excessive albumin administration.
Drug Binding: Changes in albumin levels can affect the binding and efficacy of drugs.
Globulins
Structure:
Globulins are a diverse group of proteins with varying structures, generally larger than albumin. They are divided into alpha (α), beta (β), and gamma (γ) globulins based on their electrophoretic mobility.
Function:
Alpha (α) Globulins:
α1-antitrypsin: Inhibits proteases, protecting tissues from enzymes during inflammation.
Haptoglobin: Binds free hemoglobin released from red blood cells, preventing kidney damage and iron loss.
Beta (β) Globulins:
Transferrin: Transports iron in the blood, essential for hemoglobin synthesis.
Complement proteins: Part of the immune system, they enhance the ability of antibodies and phagocytic cells to clear microbes and damaged cells.
Gamma (γ) Globulins:
Immunoglobulins (IgG, IgA, IgM, IgE, IgD): Serve as antibodies, crucial for immune defense against pathogens.
Clinical Significance:
Hypogammaglobulinemia: Low levels of immunoglobulins, leading to increased susceptibility to infections (e.g., in primary immunodeficiencies).
Hypergammaglobulinemia: Elevated levels, often seen in chronic infections, autoimmune diseases, or multiple myeloma.
Transferrin Saturation: Abnormal levels can indicate iron deficiency or overload (as in hemochromatosis).
Fibrinogen
Structure:
Fibrinogen is a large glycoprotein with a molecular weight of about 340 kDa. It is composed of three pairs of polypeptide chains (α, β, γ) linked by disulfide bonds.
Function:
Blood Clotting: Fibrinogen is converted by thrombin into fibrin during the clotting process, forming a stable clot that prevents excessive blood loss during injury.
Clinical Significance:
Hypofibrinogenemia: Low fibrinogen levels can lead to bleeding disorders, making it difficult to form clots (as seen in liver disease, disseminated intravascular coagulation, or congenital deficiencies).
Hyperfibrinogenemia: Elevated levels are associated with an increased risk of cardiovascular diseases, as fibrinogen is an acute-phase reactant that rises in inflammation.
Dysfibrinogenemia: Genetic mutations can lead to abnormal fibrinogen that either does not function properly or forms clots too easily, resulting in either bleeding or thrombosis.
C-Reactive Protein (CRP)
Structure
Composition: C-Reactive Protein (CRP) is a pentameric protein, meaning it consists of five identical subunits. Each subunit has a molecular weight of approximately 23 kDa, making the total molecular weight about 115 kDa.
Shape: CRP has a disc-like structure with calcium-binding sites that are essential for its function.
Function
Acute-Phase Reactant: CRP is one of the major acute-phase proteins, meaning its levels rise rapidly in response to inflammation, infection, or tissue injury.
Immune Response: CRP plays a role in the immune system by binding to phosphocholine on the surface of dead or dying cells and some types of bacteria, thereby activating the complement system. This leads to the opsonization of pathogens and dead cells, promoting their clearance by phagocytes.
Inflammation Marker: CRP levels are widely used as a marker of inflammation in clinical settings. It is highly sensitive but not specific, meaning it indicates the presence of inflammation but not its cause.
Clinical Significance
Inflammatory Diseases: Elevated CRP levels are seen in various inflammatory conditions, including bacterial infections, autoimmune diseases like rheumatoid arthritis, and inflammatory bowel disease (IBD).
Cardiovascular Risk: CRP is also used as a marker for cardiovascular risk. High-sensitivity CRP (hs-CRP) tests can help predict the risk of cardiovascular events like myocardial infarction and stroke, especially in individuals with otherwise normal cholesterol levels.
Monitoring Treatment: CRP levels are often monitored to assess the effectiveness of treatments for infections or inflammatory diseases. A decrease in CRP levels generally indicates that the treatment is working.
Transferrin
Structure
Composition: Transferrin is a glycoprotein with a molecular weight of about 76-80 kDa. It has two lobes, each capable of binding one iron ion (Fe3+).
Binding Sites: The protein has specific binding sites for iron, which allows it to transport iron through the blood safely.
Function
Iron Transport: Transferrin binds and transports iron ions in the blood, delivering them to cells, particularly in the bone marrow, where iron is used for hemoglobin synthesis.
Iron Homeostasis: It helps regulate iron levels in the body by binding excess iron and preventing free iron from catalyzing the formation of free radicals, which can damage cells.
Recycling: Transferrin also recycles iron from old or damaged red blood cells by transporting it back to the bone marrow or liver.
Clinical Significance
Iron Deficiency Anemia: In cases of iron deficiency, transferrin levels increase as the body tries to capture more iron. However, the transferrin saturation (the percentage of transferrin bound to iron) decreases because there is less iron available to bind.
Iron Overload: Conditions like hemochromatosis, where there is too much iron in the body, lead to increased transferrin saturation. High iron levels can deposit in organs, leading to damage.
Total Iron-Binding Capacity (TIBC): Transferrin levels are indirectly measured as part of the TIBC test, which helps diagnose and manage iron-related disorders. High TIBC suggests iron deficiency, while low TIBC may indicate chronic diseases, malnutrition, or iron overload conditions.
Chronic Disease: Inflammatory conditions can lead to a decrease in transferrin levels (a condition called hypoferremia of chronic disease), which is part of the body’s defense mechanism to reduce iron availability to pathogens.
Genetic Deficiencies of plasma proteins
Analbuminaemia
Bruton’s agammaglobulinaemia
Afibrinogenaemia
Analbuminaemia
Inherited disorder in which albumin is very low or completely absent.
Defect is in the albumin synthesis.
There is usually associated raised levels of plasma lipids and lipoproteins, which is probably secondary defect in lipid transport.
All globulin fractions occur in increased concentration.
Bruton’s agammaglobulinaemia
An inherited disorder, X-linked recessive traits.
Differentiation of B-lymphocytes to plasma cells is defective leading to lack of plasma cells in circulating blood.
There is absence of γ-globulins or γ-globulins level are very low, and lacks humoral immunity and susceptible to bacterial infections.
Afibrinogenaemia
An inherited disorder characterized by genetic defect in fibrinogen formation.
Inherited as a non-X-linked recessive trait
Characterized by absence of fibrinogen or very low level of fibrinogen.
Blood clotting mechanism is hampered and thus there may be uncontrollable haemorrhages.