Synthesis of Carboxylic Acids
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
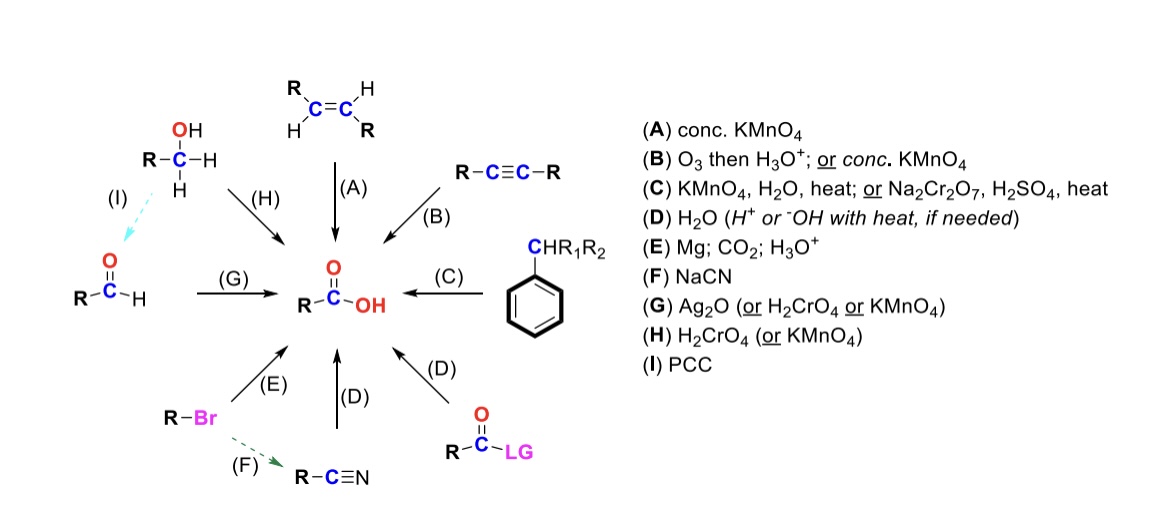
conc. KMnO4
Alkene is oxidized into carboxylic acid
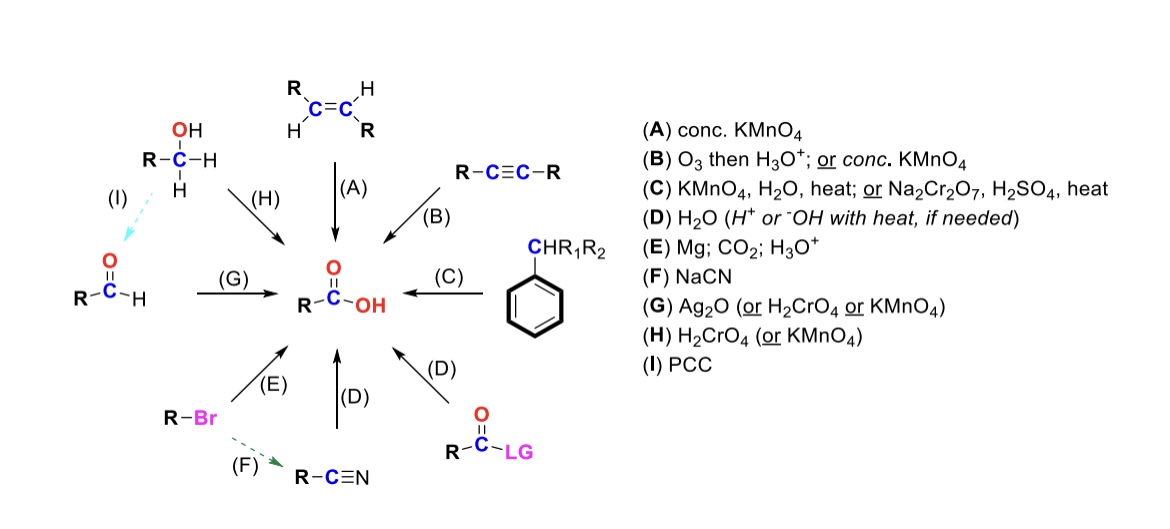
O3, H3O+ ( or conc. KMnO4 )
Internal alkyne is turned into a carboxylic acid. Terminal alkyne is turned into a carboxylic acid and a formic acid
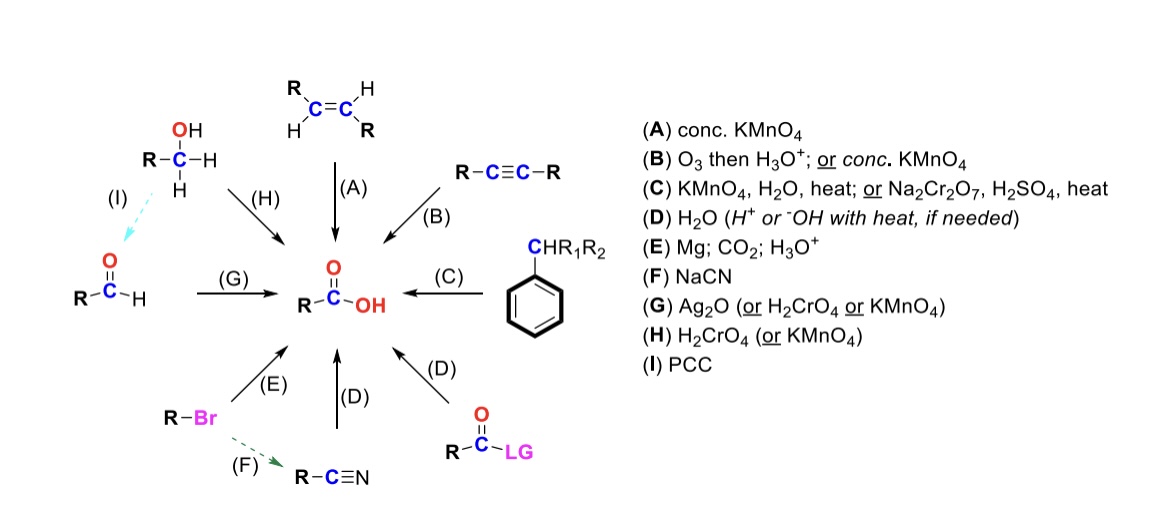
KMnO4, H2O, heat ( or KMnO4, NaOH then H3O+ )
CH2-R on a benzene is turned into a carboxylic acid
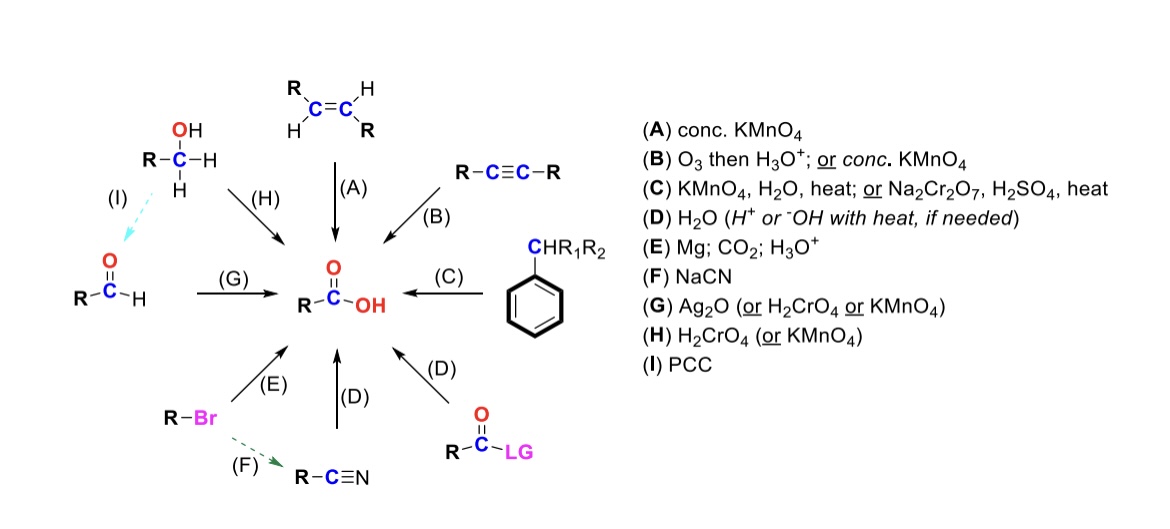
H2O ( or H+ or OH- with heat if needed )
Leaving group is displaced from carbonyl C and turned into a carboxylic acid
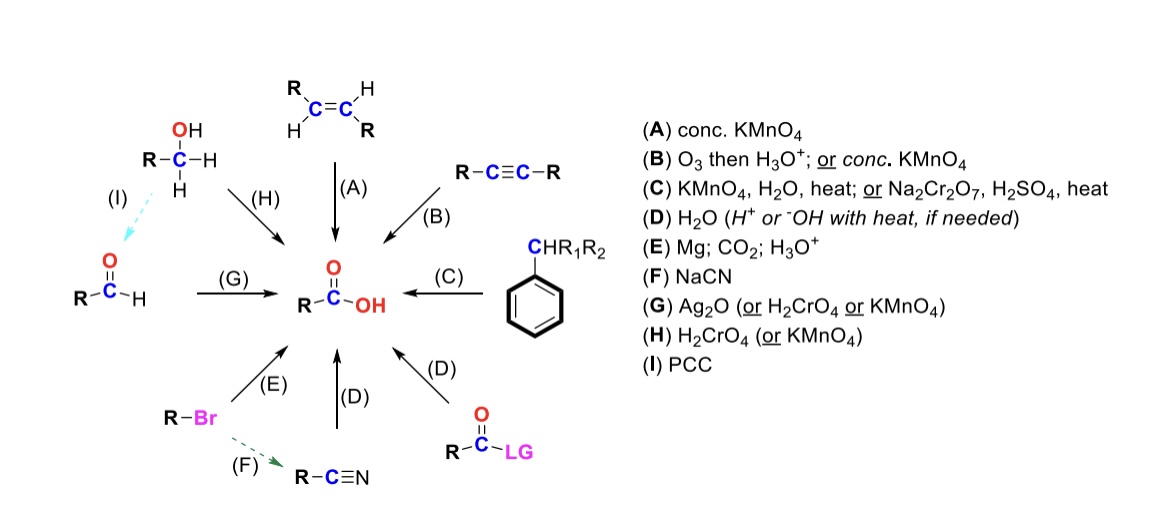
Mg, CO2, H3O+
R-Br is turned into a Grignard reagent (R-MgBr). The R group then attacks CO2. One of the O’s are protonated to form the carboxylic acid
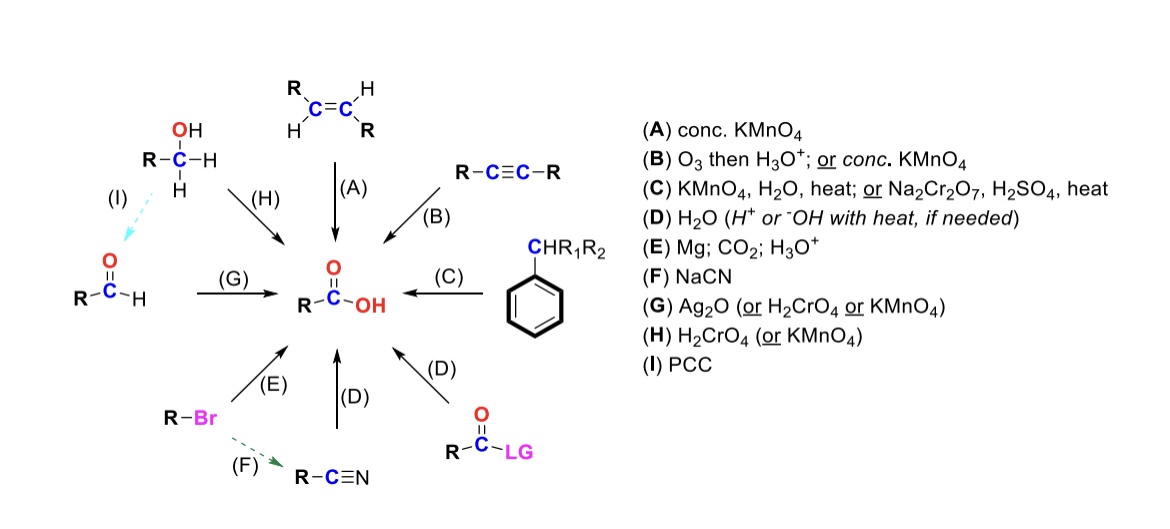
Nitrile → Carboxylic acid
NaCN, H2O (H+ or OH- with heat if needed)
Nitrile (-CN) displaces LG. Then it reacts with H2O to form a carboxylic acid
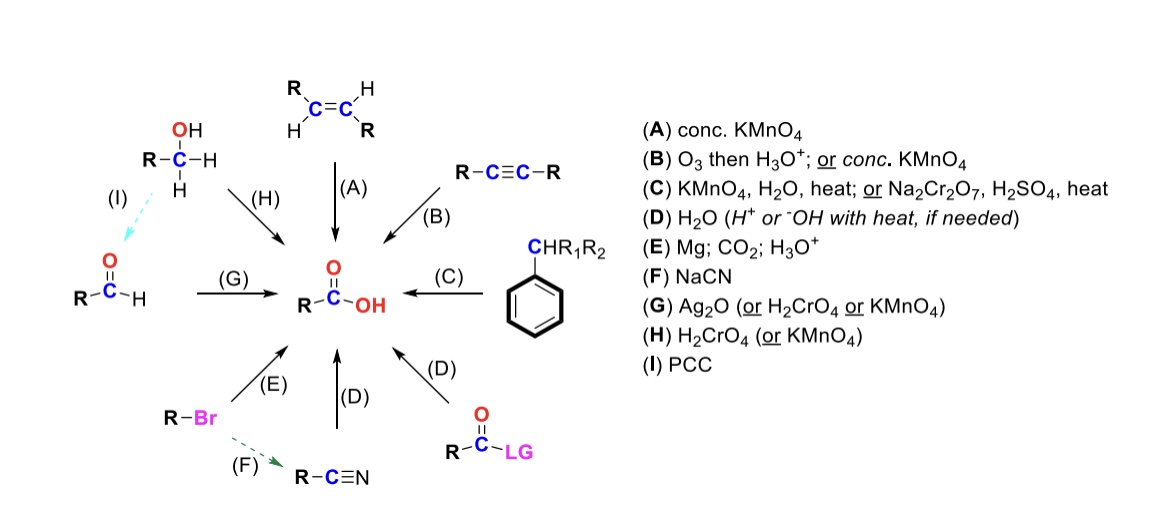
Ag2O
Aldehyde is turned into a carboxylic acid
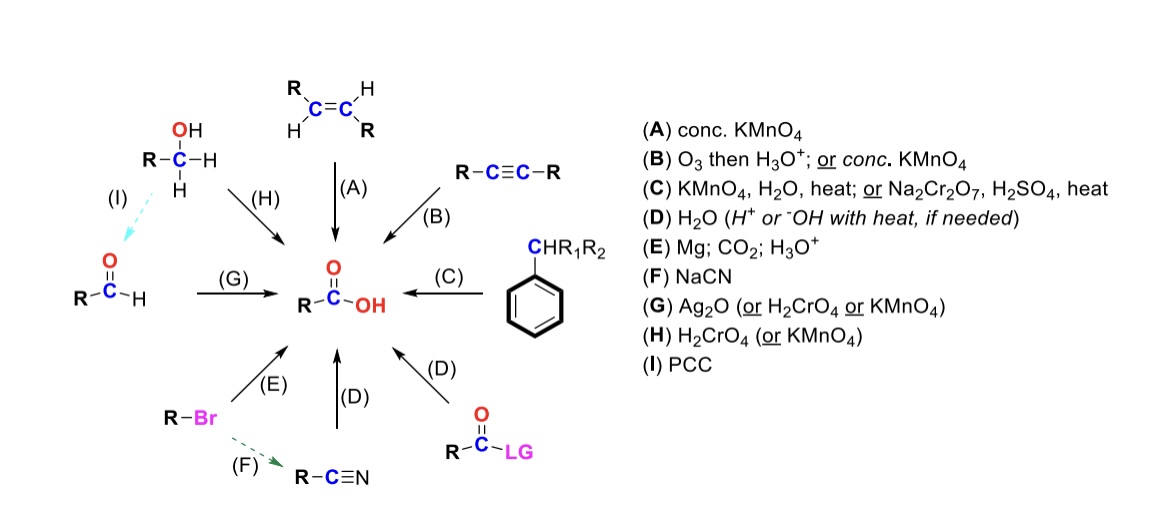
H2CrO4 ( or KMnO4 )
Primary alcohol is turned into a carboxylic acid
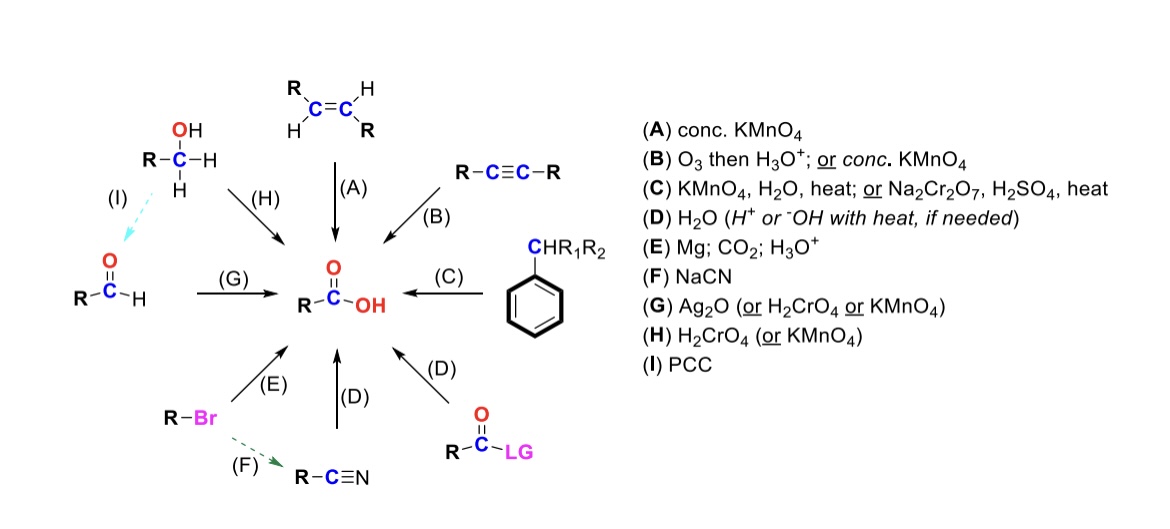
PCC
Primary alcohol is turned into an aldehyde. It can be furthered oxidized into a carboxylic acid w/ Ag2O