CHEM Titrations + Ksp + Buffers
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Titrations are used to find an unknown by adding a
known
What’s the endpoint?
When moles of acid = moles of base
Titrations can be made with any combination of strong/weak acids and bases EXCEPT for…
Weak acid + Weak base
Titrating a strong acid with strong base means start and end points are
start at low pH and end at high pH
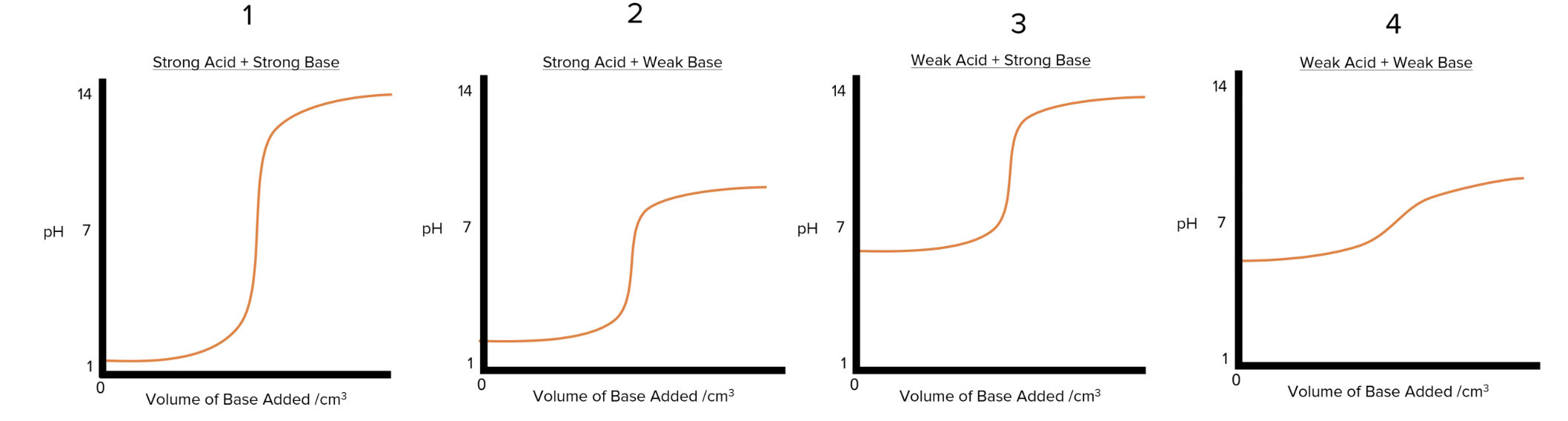
point of inflection =
equivalence point
OH is on the top half of the graph (above 7) and H+ is on the
bottom half (below 7)
At the half equivalence point pH =
pKa
when the pH is lower than the pKa, that means there is more
HA (acid) around
buffer region is around the what point?
½ equivalence point
Solubility is defined as:
How much of a compound will dissolve in a solvent to make a solution (g/L)
Molar solubility is the….
maximum concentration of a compound in a solution (M)
Saturate solution
solutions at maximum solubility
In saturated solutions concentration
= molar solubility
In unsaturated solutions concentration
< molar solubility
Unsaturated solutions…
not at maximum solubility
Supersaturated solution
above maximum solubility
supersaturated solutions have concentration
> molar solubility
Ksp indicates whether a compound is
soluble or insoluble
Ksp >> 1
is soluble (favoring aqueous products)
Ksp << 1
is insoluble (favoring solid products)
Ksp is unitless T/F
True
If Ksp > Q does a precipitate form?
no precipitate forms (forward direction)
If Ksp < Q does a precipitate form?
precipitate forms (backwards direction)
The value with the ________ Ksp will precipitate first
smallest
Smallest Ksp =
least soluble
Largest Ksp =
most soluble
Common ion effect says that
the solubility of an ionic compound is decreased in a solution that contains a common ion
adding more of a common ion will shift the equilibrium toward…
the side without it. This usually suppresses ionization and reduces solubility or acidity.
larger pKa =
smaller Ka
Ka to pKa =
-log(Ka)
pKa to Ka =
10-pKa
pKa =
-log(Ka)
If the pKa and pH are close together, then that makes a ______ buffer
good