microbio chapter 10
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
107 Terms
What is metabolism?
All chemical reactions and physical workings of the cell.
What is anabolism?
Biosynthesis: the synthesis of cell molecules and structures; it requires energy input.
What is catabolism?
The breakdown of larger molecules into smaller ones, which releases energy.
What are the main accomplishments of metabolism?
1. Assembles smaller molecules into macromolecules using ATP (anabolism)
2. Breaks down macromolecules to release energy (catabolism)
3. Collects and spends energy in the form of ATP or heat
model of metabolism
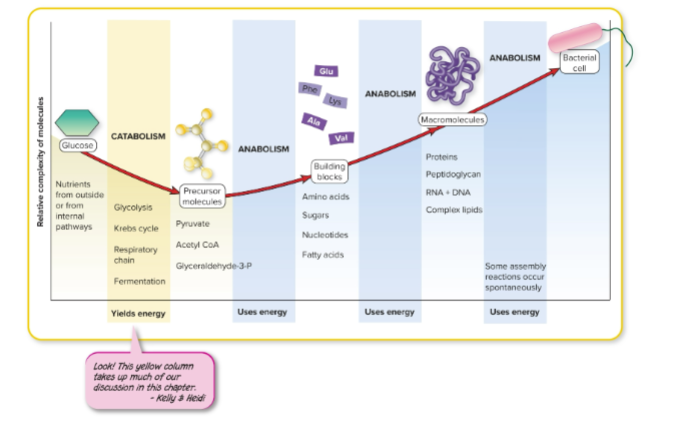
What is a catalyst?
A substance that speeds up a chemical reaction without being consumed or becoming part of the product.
How do enzymes act as catalysts in chemical reactions?
: They overcome activation energy by:
Increasing thermal energy to speed up molecular movement
Increasing reactant concentration to enhance collisions
Adding a catalyzing substance to facilitate the reaction
What are substrates?
Reactant molecules upon which enzymes act.
How do enzymes interact with substrates?
Enzymes bind to substrates and participate in changes to the substrate, without becoming part of the product or being consumed, and can function repeatedly.
What are the two main types of enzymes based on structure?
1. Simple enzymes – consist of protein alone
2. Conjugated enzymes – consist of protein and a nonprotein component
What is a holoenzyme?
The complete, functional form of a conjugated enzyme, including both protein and nonprotein parts.
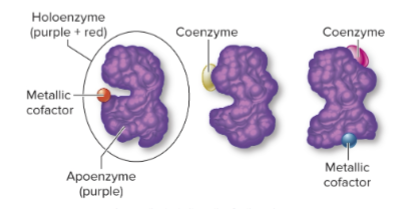
What is an apoenzyme?
The protein portion of a holoenzyme, inactive without its cofactor.
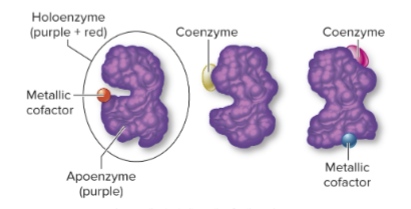
What is a cofactor?
The nonprotein portion of a holoenzyme, which can be:
Organic molecule (coenzyme)
Inorganic molecule (metal ion)
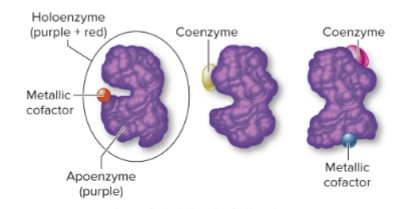
Why are cofactors important?
They assist the enzyme in performing its catalytic activity.
enzyme substrate reactions
The process in which an enzyme binds to its substrate and converts it into a product while remaining unchanged itself.
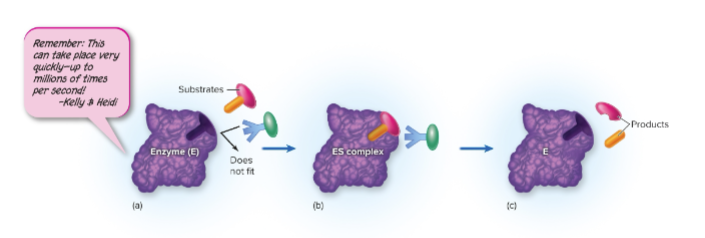
What is the active site of an enzyme?
The specific region of an enzyme where the substrate binds and the reaction takes place.
How do enzymes and substrates fit together?
Through either:
Lock-and-key model: Substrate fits perfectly into the enzyme’s active site.
Induced-fit model: Enzyme slightly changes shape to fit the substrate more snugly.
What happens during the enzyme–substrate reaction?
1. Substrate binds to the enzyme’s active site.
2. Enzyme stabilizes the transition state, lowering activation energy.
3. Substrate is converted into product(s).
4. Product(s) are released, and the enzyme is free to catalyze another reaction.
Can an enzyme be reused after a reaction?
Yes, enzymes are not consumed and can catalyze the same reaction repeatedly.
What factors can affect enzyme–substrate reactions?
- Temperature
pH
Substrate concentration
Enzyme concentration
Presence of inhibitors or activators
Lactase (β-D-galactosidase)
Class: Hydrolase
Substrate: Lactose
Action: Breaks lactose down into glucose and galactose
Penicillinase (β-lactamase)
Class: Hydrolase
Substrate: Penicillin
Action: Hydrolyzes the beta-lactam ring of penicillin
DNA polymerase (DNA nucleotidyl-transferase)
Class: Transferase
Substrate: DNA nucleosides
Action: Synthesizes a strand of DNA using the complementary strand as a template
Lactate dehydrogenase
Class: Oxidoreductase
Substrate: Pyruvic acid
Action: Catalyzes the conversion of pyruvic acid to lactic acid
Oxidase (Cytochrome oxidase)
Class: Oxidoreductase
Substrate: Molecular oxygen (O₂)
Action: Catalyzes the reduction of O₂ (addition of electrons and hydrogen)
What is oxidation?
The loss of electrons; a compound that loses electrons is oxidized.
What is reduction?
The gain of electrons; a compound that gains electrons is reduced.
What coenzymes act as electron carriers in redox reactions?
NAD (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide).
What are constitutive enzymes?
Enzymes that are always present in relatively constant amounts, regardless of the cellular environment.
What are regulated enzymes?
Enzymes whose production is turned on (induced) or off (repressed) in response to changes in substrate concentration
constitutive and regulated enzymes
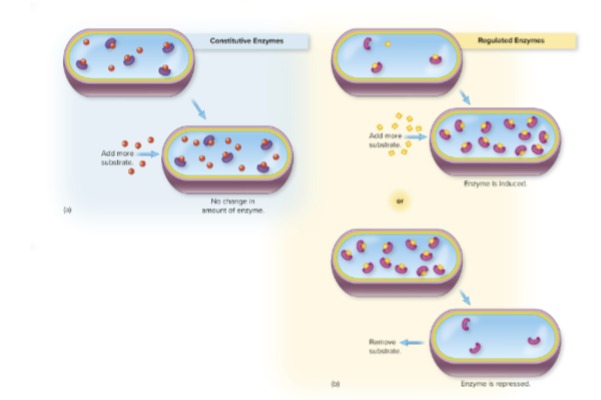
Why is enzyme regulation important?
It allows the cell to conserve energy and resources by producing enzymes only when needed.
How do microbial enzymes contribute to disease?
Pathogens secrete exoenzymes that help them avoid host defenses or promote multiplication in tissues; these are virulence factors and some function as toxins.
Give examples of microbial exoenzymes involved in pathogenicity.
Streptokinase, streptolysin, elastase, collagenase, lipase, penicillinase
What happens when enzymes are sensitive to changes from normal conditions?
They become chemically unstable (labile), losing functionality.
What is denaturation of enzymes?
Breaking of weak bonds that maintain enzyme shape by heat, pH changes, or chemicals, which distorts the enzyme and prevents substrate binding, blocking metabolic reactions
possibly lead to cell death
What is a metabolic pathway?
A multistep series of reactions where each step is catalyzed by an enzyme, the product of one reaction is the substrate for the next, and pathways can be branched or cyclic.
pathways interconnected and merge at many sites
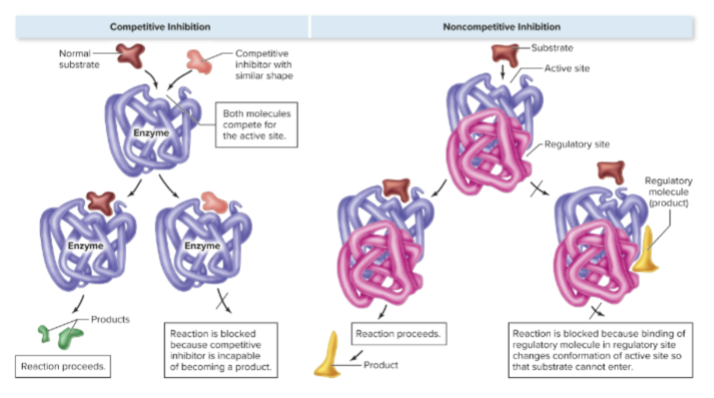
What is competitive inhibition?
A molecule resembling the substrate (a mimic) occupies the enzyme’s active site, preventing the substrate from binding, effectively shutting down the enzyme due to being unable to act on the inhibitor
What is noncompetitive inhibition?
Inhibition that occurs when a molecule binds to a site other than the active site (regulatory site) on the enzyme, altering its shape and reducing activity.
regulated by the binding of molecules other than the substrate to the regulatory site
often the regulatory molecule is the product of the enzymatic reaction
provides negative feedback that slows enzyme activity once a certain concentration of product is reached
How does noncompetitive inhibition often function in cells?
The regulatory molecule is often the product of the enzymatic reaction, providing negative feedback to slow enzyme activity once product concentration is sufficient.
competitive and noncompetitive inhibition visual
What is the difference between feedback inhibition and enzyme repression?
- Feedback inhibition (noncompetitive): Slows enzyme activity directly, immediate effect.
Enzyme repression: Stops further synthesis of the enzyme at the genetic level when the end product is in excess; longer response and more enduring effect.
Why is enzyme repression important?
It conserves energy and resources by preventing unnecessary enzyme production once enough product is made.
enzyme repression visual
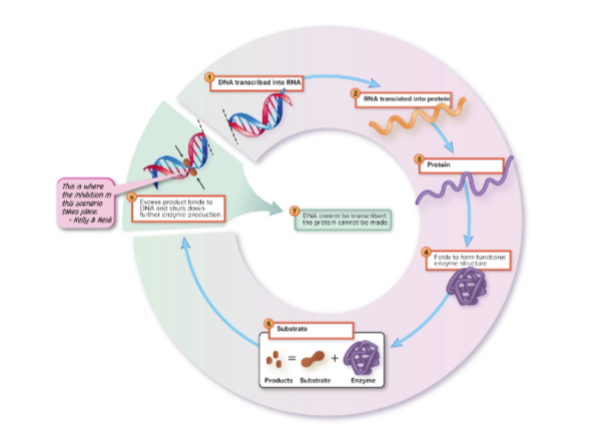
What is enzyme induction?
The process where enzymes are synthesized only when their substrate is present; it is the inverse of enzyme repression.
synthesis of an enzyme is induced by its substrate
What are exergonic reactions?
Reactions that release energy as they go forward, making it available for cellular work.
What are endergonic reactions?
Reactions that require an input of energy to proceed.
How are exergonic and endergonic reactions often related?
They are often coupled, with energy released from exergonic reactions used to drive endergonic reactions.
What is a redox reaction?
A chemical reaction involving oxidation (loss of electrons) and reduction (gain of electrons), always occurring in pairs called redox pairs.
What are the roles of electron donors and acceptors in redox reactions?
- Electron donor: loses electrons (oxidized)
Electron acceptor: gains electrons (reduced), storing energy that can be used to phosphorylate ADP or other compounds.
Which coenzyme carriers are important in cellular redox reactions?
NAD (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide).
Why are newly reduced compounds energetic?
They have more energy than in their oxidized state, which can be captured to make high-energy molecules like ATP from phosphorylation of ADP
stores energy in a high-energy molecule
What is NAD and its role?
most common electron carrier; carries hydrogens and a pair of electrons from dehydrogenation reactions. Reduced NAD is NADH + H⁺.
What is FAD and its role?
electron carrier that accepts electrons and hydrogens. Reduced FAD is FADH
What is NADP?
NAD phosphate, another electron carrier similar to NAD, primarily used in anabolic reactions like photosynthesis.
What happens in catabolic pathways?
Electrons are extracted from molecules and carried through redox reactions to a final electron acceptor.
What is the final electron acceptor in aerobic metabolism?
Oxygen (O₂)
What is the final electron acceptor in anaerobic metabolism?
Some other inorganic or organic compound (not O₂).
What is ATP (adenosine triphosphate) made of?
Three-part molecule:
Adenine (nitrogen base)
Ribose (5-carbon sugar)
Three phosphate groups bonded to ribose
Why is ATP high-energy?
The negative charges on the last two phosphate groups repel each other, creating strain; removal of phosphates releases free energy.
what does ATP look like?
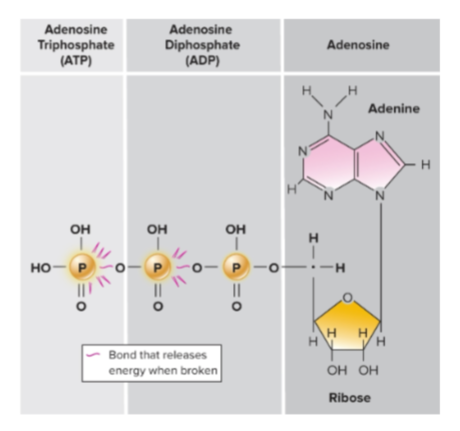
What is ATP?
The primary energy currency of the cell; must be continuously replenished as it is used in chemical reactions.
What is substrate-level phosphorylation?
Generation of ATP by transferring a phosphate group from a phosphorylated compound directly to ADP.
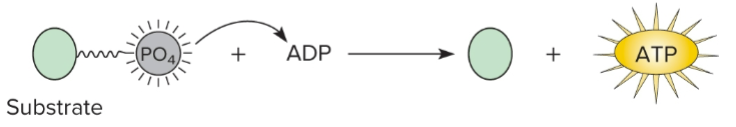
What is oxidative phosphorylation?
ATP production through a series of redox reactions during the final phase of the respiratory pathway.
What is photophosphorylation?
ATP formation through sunlight-driven reactions in phototrophic organisms.
How are ATP utilization and replenishment related?
They occur in a continuous cycle, ensuring the cell always has energy available for chemical reactions.
What are the three basic catabolic pathways?
Aerobic respiration, anaerobic respiration, fermentation
What is the most common pathway to break down glucose?
Glycolysis
overview of the three main pathways of catabolism
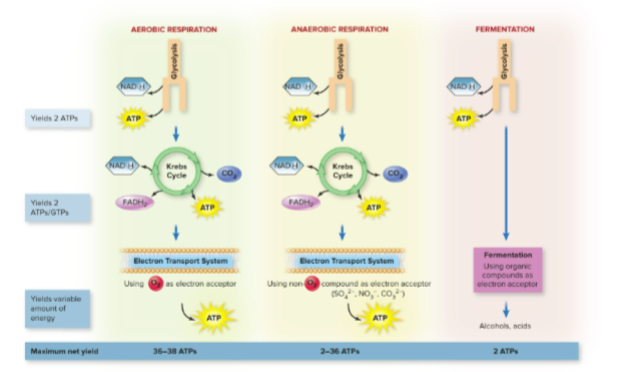
What is the final electron acceptor in aerobic respiration?
Molecular oxygen (O₂)
What is the final electron acceptor in anaerobic respiration?
Inorganic molecules like NO₃⁻, SO₄²⁻, or CO₃²⁻
Which pathways are used in both aerobic and anaerobic respiration?
Glycolysis, Krebs cycle, and the respiratory chain
What is the maximum ATP yield for aerobic versus anaerobic respiration?
- Aerobic: 36–38 ATP
- Anaerobic: 2–36 ATP
What is fermentation?
A pathway used by facultative and aerotolerant anaerobes that:
Uses only glycolysis
Does not require oxygen
Uses organic compounds as electron acceptors
Why is fermentation less efficient than respiration?
It produces much less ATP (only 2 per glucose) because it does not use the Krebs cycle or the electron transport chain.
What is aerobic respiration?
A series of enzyme-catalyzed reactions where electrons are transferred from fuel molecules to oxygen, the final electron acceptor.
the principal energy-yielding pathway for aerobic heterotrophs and provides ATP and metabolic intermediates.
Why is glucose a good fuel for cells?
Glucose and other carbohydrates are readily oxidized, excellent hydrogen and electron donors, and provide electrons for energy transfer.
What are the end products of glucose oxidation in aerobic respiration?
ATP (energy-rich), carbon dioxide, and water (energy-poor).
What is glycolysis?
The enzymatic conversion of glucose to pyruvic acid, producing a small amount of ATP anaerobically. It is the first step of aerobic respiration and a key metabolic intermediate.
Why is pyruvic acid important?
It is an essential intermediary metabolite that feeds into the Krebs cycle and other metabolic pathways.
summary of glycolysis
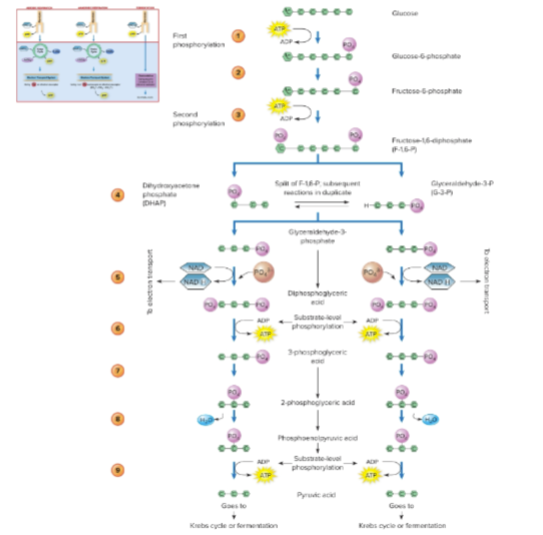
What happens to pyruvic acid before it enters the Krebs cycle?
Pyruvic acid is converted to acetyl coenzyme A (acetyl CoA), releasing the first CO₂ and reducing NAD⁺ to NADH.
oxidation reaction releases the first CO2 molecule
a cluster of enzymes and coenzyme A dehydrogenate pyruvic acid to a 2-carbon acetyl group
What is the role of NADH formed in the conversion of pyruvate to acetyl CoA?
NADH is shuttled to the electron transport system to produce ATP.
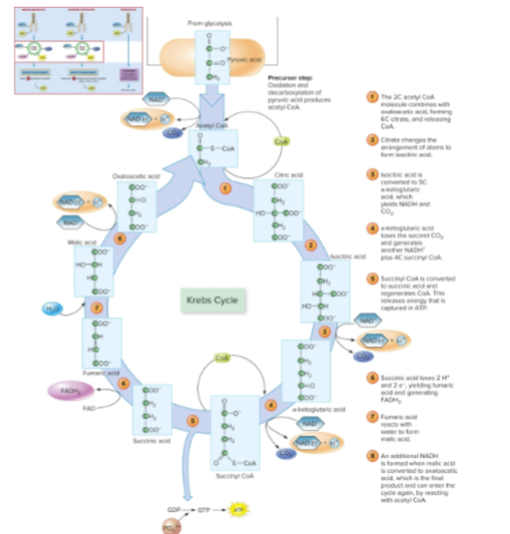
How many times does the Krebs cycle run per glucose molecule?
Twice, because glycolysis produces two pyruvate molecules per glucose.
What is the main function of the Krebs cycle?
To transfer energy stored in acetyl CoA to electron carriers (NAD⁺ and FAD) by reducing them.
What are the main products of the Krebs cycle per glucose molecule?
- Reduced NADH (and FADH₂)
2 ATP produced via substrate-level phosphorylation
reactions of a single turn of the Krebs cycle visual
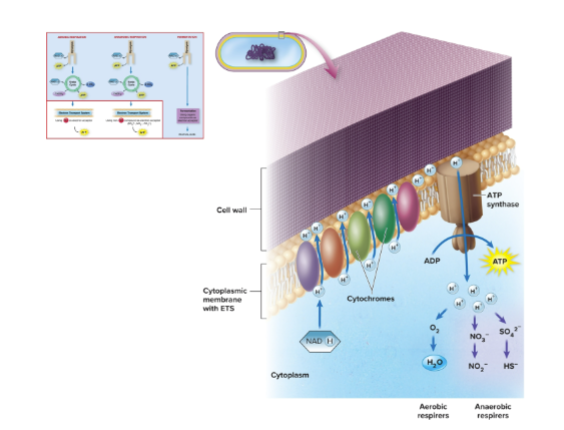
What is the electron transport system (ETS)?
A chain of special redox carriers that receives electrons from NADH (and FADH₂), passing them sequentially to transfer energy and pump hydrogens across the membrane.
What is the final electron acceptor in the ETS of aerobic organisms?
Oxygen (O₂), which combines with electrons and protons to form water.
What is the purpose of electron flow in the ETS?
The flow of electrons drives the active transport of hydrogen ions (protons) outside the membrane, creating a proton gradient used to generate ATP.
What is the typical sequence of electron carriers in most aerobic organisms?
1. NADH dehydrogenase
2. Flavin mononucleotide (FMN)
3. Coenzyme Q (ubiquinone)
4. Cytochrome b
5. Cytochrome c₁
6. Cytochrome c
7. Cytochromes a and a3
What is the relationship between the ETS and oxidative phosphorylation?
The proton gradient generated by electron flow in the ETS drives ATP synthesis through oxidative phosphorylation.
the electron transport system and oxidative phosphorylation in bacterial membranes (visual)
The Electron Transport System on the Inner Membrane of the Mitochondrial Cristae (visual)
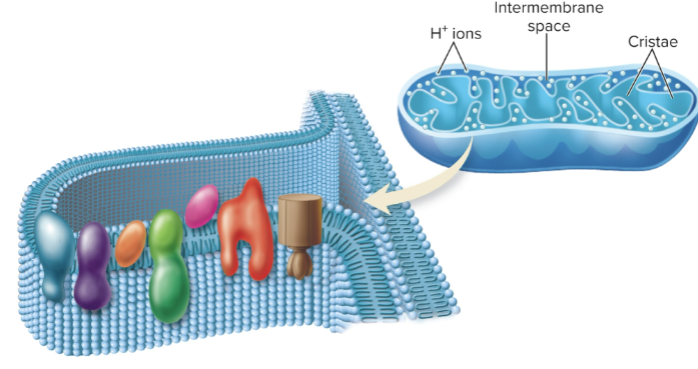
What is ATP synthase?
An enzyme stationed along the membrane near ETS carriers that captures energy from electron transport to synthesize ATP.
What is oxidative phosphorylation?
The coupling of ATP synthesis to electron transport; each NADH entering the ETS typically produces 3 ATP, while FADH₂ produces 2 ATP due to entering at a lower-energy point.
What is chemiosmosis?
As the ET carriers shuttle electrons, hydrogen ions are pumped into the periplasmic space or the space between the cell wall and cytoplasmic membrane
The process in which hydrogen ions (protons) are pumped across the membrane by ETS carriers, creating a proton concentration gradient.
What is the proton motive force (PMF)?
The electrochemical gradient of protons across the membrane:
Outside membrane: positive (+)
Inside membrane: negative (−)
This stores potential energy that drives ATP synthesis via ATP synthase.
How do protons return to the cytoplasm to produce ATP?
Protons diffuse back through ATP synthase, releasing energy that drives the phosphorylation of ADP to ATP.